extracellular vesicles
Want to get insights into potency of extracellular vesicles / exosomes produced by specific cells? Interested in EV enrichment, purification, characterization?
why get services from Evercyte
Evercyte has a highly experienced scientific team
experience
benefit from our know-how of > 5 years in isolation / production and characterization of extracellular vesicles from human cells and tissues
quality
highest quality standards in characterization of extracellular vesicles following MISEV2023 criteria (minimal information for studies of extracellular vesicles 2023, released by the International Society for Extracellular Vesicles ISEV)
one-stop-shop
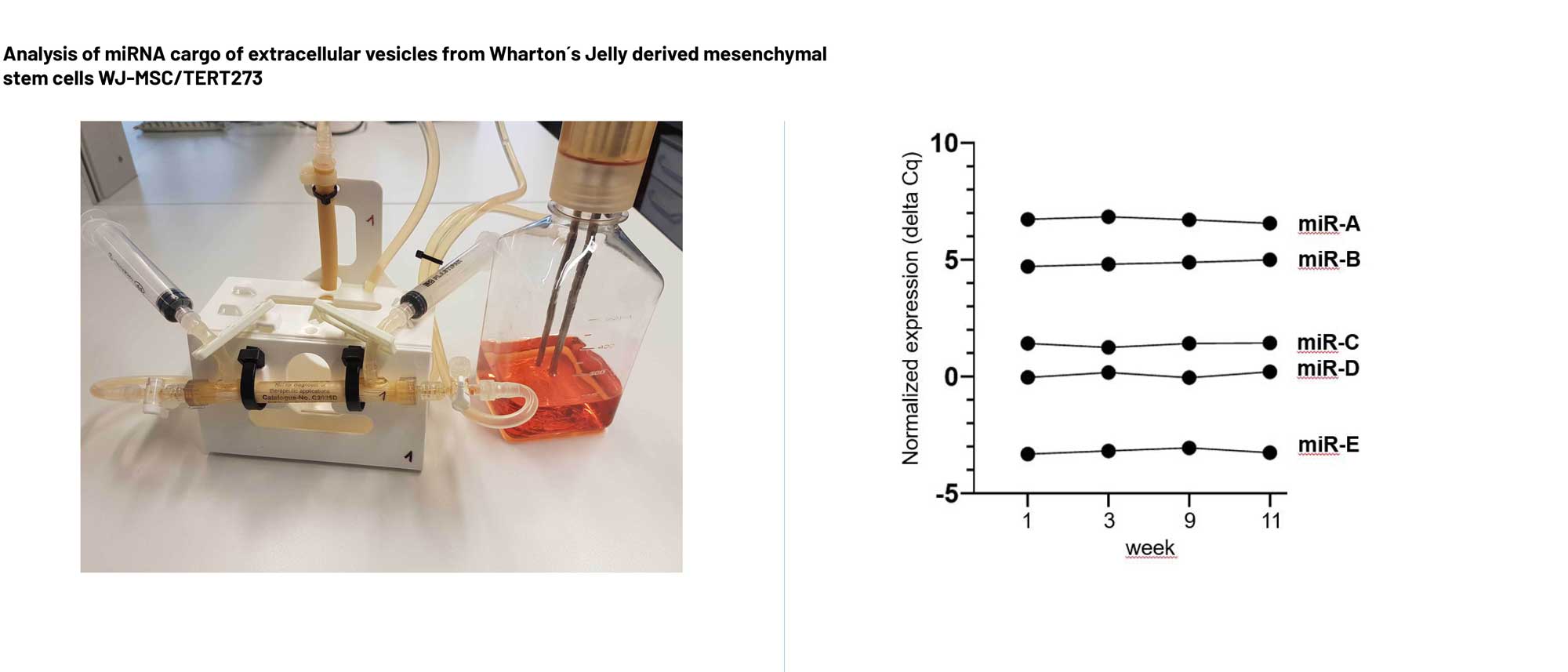
Evercyte as sole contracting partner to run even complex projects, from production of extracellular vesicles from telomerized cells or customer-derived cells, characterization of exosomes from protein content to miRNA cargo in reliable timelines
workflow
From your need to high quality extracellular vesicles
request and proposal
send us your specific request and we will come back with study design and proposal for discussion and approval
isolation / production
isolation of exosomes from whole human tissue following ethical and legal requirements or from cells of interest, enrichment and purification (tangential flow filtration, ultra-centrifugation, size exclusion chromatography)
functional testings and QC
characterization of size and number (nanoparticle tracking analysis), protein content (western blot, multiplex flow cytometry), morphology (electron microscopy), RNA cargo (RT-qPCR, small RNAseq), testing of potency (e.g. neo-angiogenic activity, anti-inflammatory and anti-fibrotic activity, skin cell migration)
why get services from Evercyte
Evercyte has a highly experienced scientific team
experience
benefit from our know-how of > 5 years in isolation / production and characterization of extracellular vesicles from human cells and tissues
quality
highest quality standards in characterization of extracellular vesicles following MISEV2018 criteria (minimal information for studies of extracellular vesicles 2018, released by the International Society for Extracellular Vesicles ISEV)
one-stop-shop
Evercyte as sole contracting partner to run even complex projects, from production of extracellular vesicles from telomerized cells or customer-derived cells, characterization of exosomes from protein content to miRNA cargo in reliable time lines
workflow
From your need to high quality extracellular vesicles
request and proposal
send us your specific request and we will come back with study design and proposal for discussion and approval
isolation / production
isolation of exosomes from whole human tissue following ethical and legal requirements or from cells of interest, enrichment and purification (tangential flow filtration, ultra-centrifugation, size exclusion chromatography)
functional testings and QC
characterization of size and number (nano particle tracking analysis), exosome protein content (western blot, multiplex flow cytometry), morphology (electron microscopy – cryo-EM), RNA cargo (RT-qPCR, small RNAseq, RNAseq), testing of potency (angiogenic, activity, anti-inflammatory activity, skin cell migration, myofibroblast activation)
customer reviews
“I have had the pleasure of working with Evercyte for the last few years. We continually rely on Evercyte because of the high-quality data that they produce, their diligent responsiveness, and their excellent customer service.”
Josh Garlich, Senior Research Scientist, Apellis Pharmaceuticals, Inc.
“Cytonus has been working with Evercyte from many years as they are a trusted partner and have always delivered the highest quality cell lines to advance our platform. We routinely draw on their expertise to meet cellular engineering challenges and they have not disappointed.”
Remo Moomiaie-Qajar, Cytonus Therapeutics, Inc.