Adipose tissue
ASC/TERT300
Evercyte’s ASC/TERT300 cell line has been developed from adipose-derived mesenchymal stromal cells, can be grown without limitations, shows typical markers and functions of MSCs and therefore represents a valuable model for studying processes such as differentiation, inflammation, tissue homeostasis and repair. In recent years evidence has accumulated that the potency of mesenchymal stromal cells at least in part is mediated by secreted extracellular vesicles (EVs). ASC/TERT300 cell line has been established under conditions that will allow production of EVs for clinical applications.
General information
Cat#: CHT-001-0300
Morphology and growth characteristics
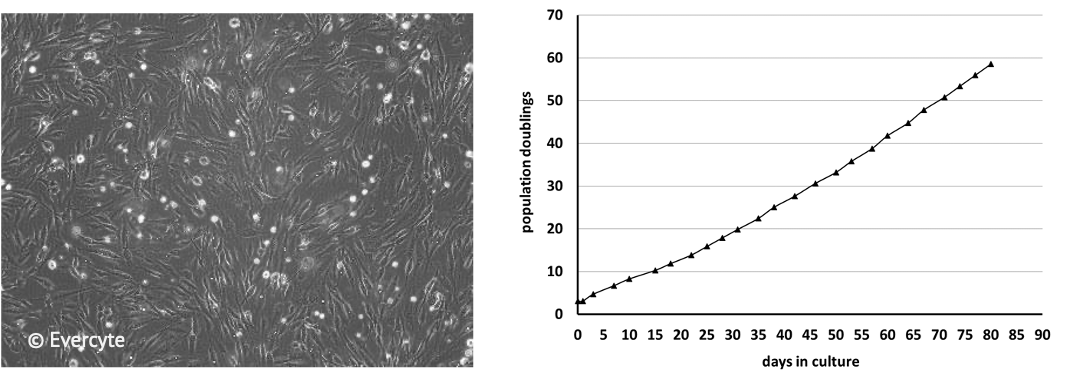
ASC/TERT300 cells can be grown for a minimum of 60 population doublings with a stable growth rate and without showing signs of growth retardation. The cells are characterized by the typical spindle-shaped mesenchymal morphology.
Expression of MSC marker proteins CD73, CD90, CD105
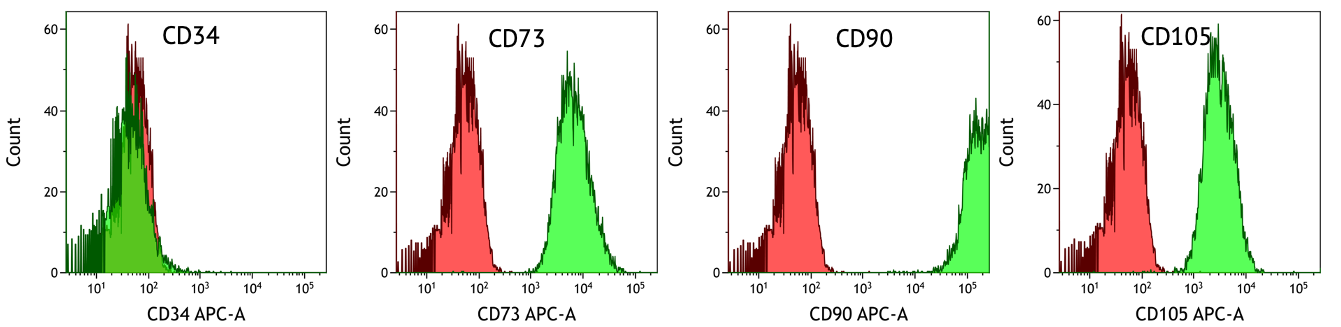
ASC/TERT300 cells homogenously express typical marker proteins of mesenchymal stem cells such as CD73, CD90 and CD105, whereas the hematopoietic stem cell marker CD34 is not expressed (green peaks). Cells stained with isotype control antibodies are used as negative control (red peaks).
Differentiation potential towards adipocytes, osteoblasts and chondrocytes
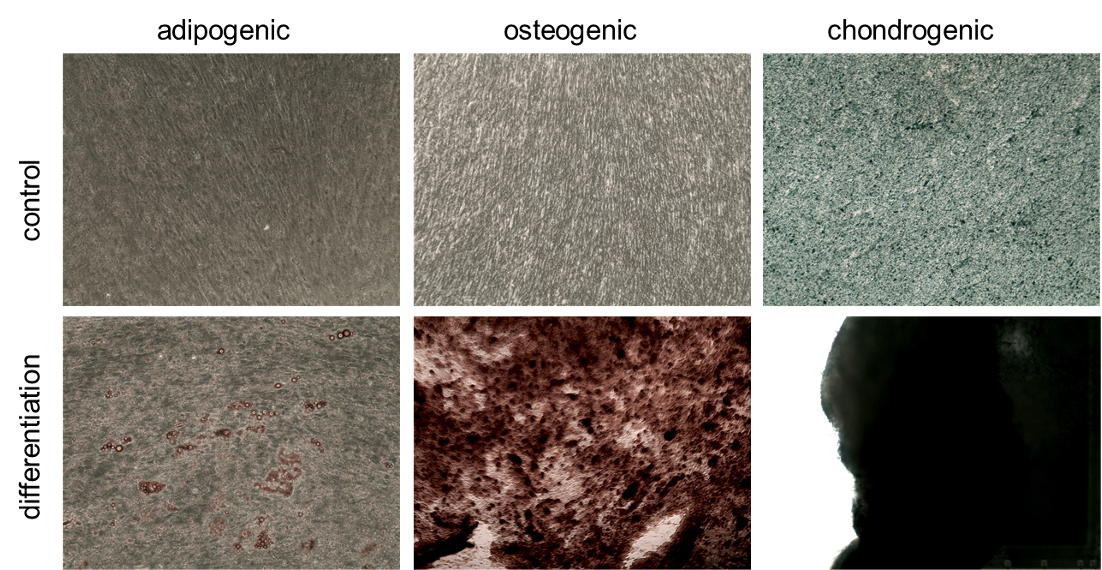
ASC/TERT300 cells can be induced to differentiate towards adipocytes (oil red O staining), osteoblasts (Alizarin red staining) and chondrocytes ( alcian blue staining).
FAQs
In vitro propagation
Mesencult – ACF Plus Culture Kit
Mesencult – ACF Plus Culture Kit including Animal Component-Free Cell Attachment (STEMCELL TECHNOLOGIES, Cat# 05448)
2 mM GlutaMAX-I (Gibco, Cat# 35050-038)
Additional material & reagents
Phosphate buffered saline (PBS) (Sigma, Cat# D8537)
CTS TrypLE Select Enzyme (Gibco, Cat# A1285901)
Passaging of cells
The new culture flasks have to be pre-coated with ACF Cell Attachment Substrate following the instructions of the manufacturer. Briefly, dilute the substrate 1:300 in PBS and transfer the diluted substrate to the culture flasks (72 µl/cm²), incubate at least 2 hours at room temperature.
Cryopreservation
Freezing medium
CryoStor(R) cell cryopreservation medium CS10 (Sigma Aldrich, Cat# C2874
Additional material & reagents
Freezing of cells
Detach the cells after having reached about 70-80 % confluence from the culture vessel by using CTS TrypLE Select Enzym solution (Protocol passaging of ASC/TERT300).
Thawing of cells
Original Evercyte cells are to be thawed in a T25 roux flask
Licence Conditions
The business concept of Evercyte is to out-license telomerized cells to our customers. The license conditions depend on whether the contract partner is a for profit or a nonprofit organization and the intended use of the cells.
Nonprofit organizations
On time payment for unlimited use: EUR 1700
Profit organizations
Pharmaceutical – chemical- cosmetic industries
Contract research organizations (CRO)
Initial license fee for 3 months: EUR 2700Annual license fee R&D: royalty based
Customer Reviews
“I have had the pleasure of working with Evercyte for the last few years. We continually rely on Evercyte because of the high-quality data that they produce, their diligent responsiveness, and their excellent customer service.”
Josh Garlich, Senior Research Scientist, Apellis Pharmaceuticals, Inc.
“Cytonus has been working with Evercyte from many years as they are a trusted partner and have always delivered the highest quality cell lines to advance our platform. We routinely draw on their expertise to meet cellular engineering challenges and they have not disappointed.”
Remo Moomiaie-Qajar, Cytonus Therapeutics, Inc.
Customer reviews
“I have had the pleasure of working with Evercyte for the last few years. We continually rely on Evercyte because of the high-quality data that they produce, their diligent responsiveness, and their excellent customer service.”
Josh Garlich, Senior Research Scientist, Apellis Pharmaceuticals, Inc.
“Cytonus has been working with Evercyte from many years as they are a trusted partner and have always delivered the highest quality cell lines to advance our platform. We routinely draw on their expertise to meet cellular engineering challenges and they have not disappointed.”
Remo Moomiaie-Qajar, Cytonus Therapeutics, Inc.

