Services
lab scale EV production
Extracellular vesicles (EVs) play an essential role in cellular communication by transporting proteins, lipids as well as nucleic acids. Evercyte offers the production of extracellular vesicles from customer-derived cells as well as from telomerized mesenchymal stem cells (MSCs) or differentiated cells. Besides production of EVs from 2D monolayer culture, protocols have been established for production of extracellular vesicles in hollow fiber bioreactor systems.
General information
Cells for EV production: Evercyte produces extracellular vesicles from any cell type of interest; please select from our broad product catalogue of telomized human cells or send us the cell line(s) you are interested in.
EVs production and stability of enriched EV preparations
hollow fiber bioreactor
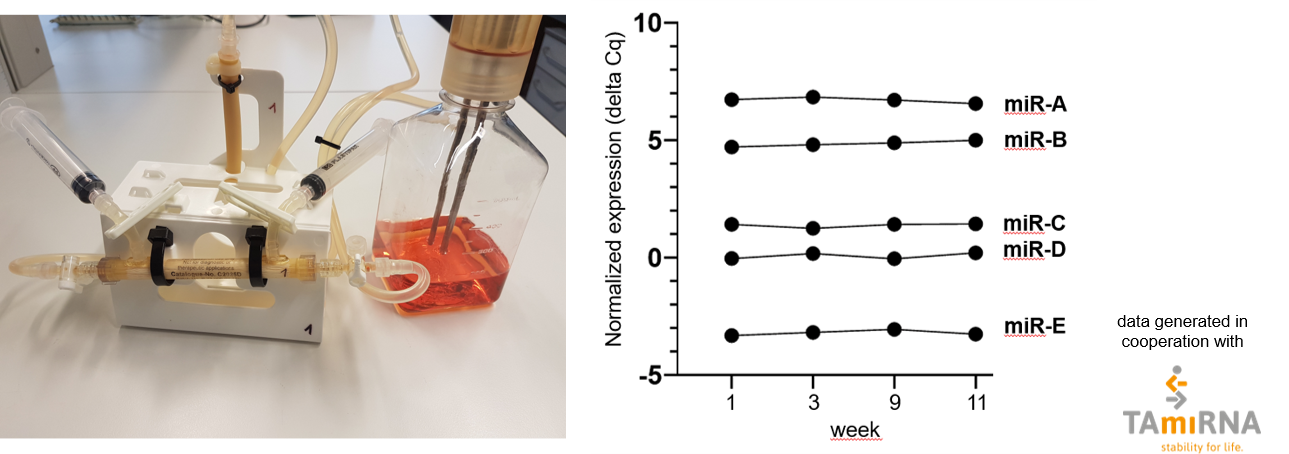
Extracellular vesicles from mesenchymal stem cells are produced in a hollow fiber bioreactor system with continuous harvests over a peroid of at least 4 months. After enrichment from supernatants using TFF, the EVs, harvested at different time points during the production phase (1, 3, 9, 11 weeks), have been characterized for their miRNA cargo. As shown in the right graph, EVs show by a similar miRNA cargo (selection of miRNAs is shown) demonstrating that the hollow fiber bioreactor system allows stable EV production.
Metabolic control of EV production process
monitoring glucose / glutamine consumtion and accumulation of metabolites
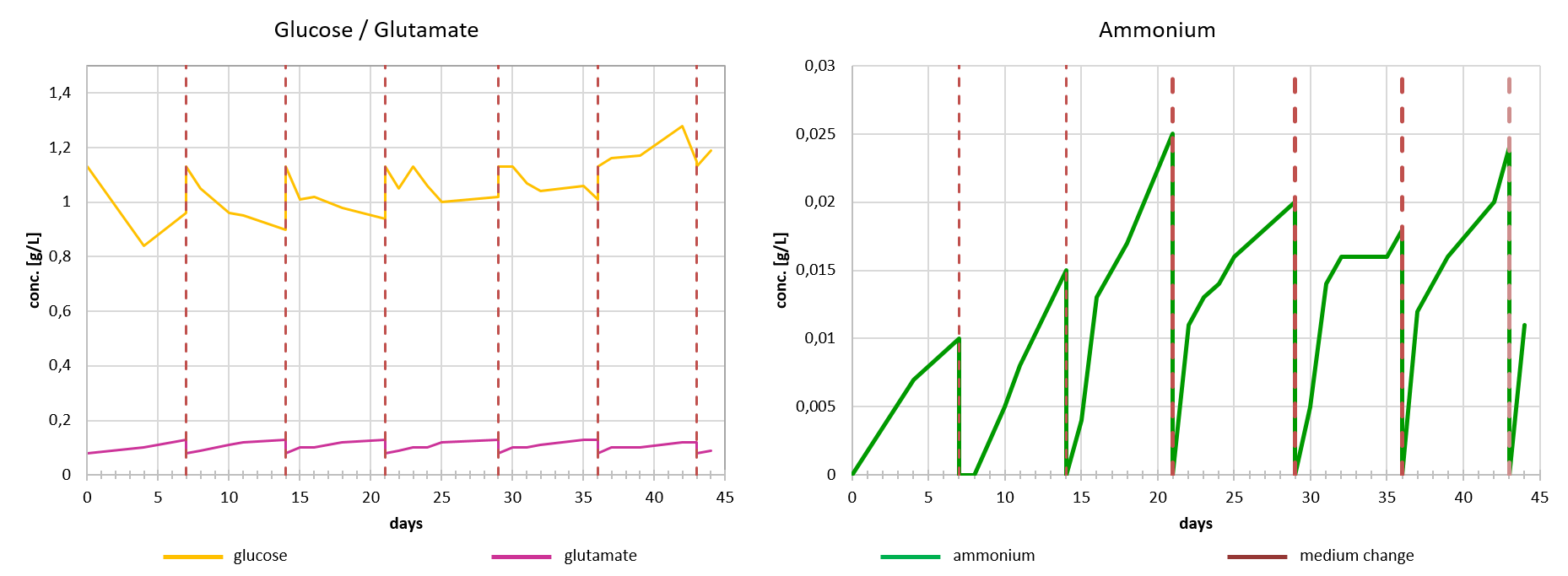
In order to ensure an optimal supply of the cells with nutrients during the production process, the consumption of glucose and glutamine is continuously monitored. An optimal feeding strategy is developed accordingly. The content of certain metabolites such as ammonium is also measured regularly.
Leaflets - extracellular vesicles
Selected publications
Gobin J, Muradia G, Mehic J, Westwood C, Couvrette L, Stalker A, Bigelow S, Luebbert CC, Bissonnette FS, Johnston MJW, Sauvé S, Tam RY, Wang L, Rosu-Myles M, Lavoie JR. (2021) Hollow-fiber bioreactor production of extracellular vesicles from human bone marrow mesenchymal stromal cells yields nanovesicles that mirrors the immuno-modulatory antigenic signature of the producer cell. Stem Cell Res Ther. 2021 Feb 12;12(1):127. https://pubmed.ncbi.nlm.nih.gov/33579358/Customer Reviews
“I have had the pleasure of working with Evercyte for the last few years. We continually rely on Evercyte because of the high-quality data that they produce, their diligent responsiveness, and their excellent customer service.”
Josh Garlich, Senior Research Scientist, Apellis Pharmaceuticals, Inc.
"Cytonus has been working with Evercyte from many years as they are a trusted partner and have always delivered the highest quality cell lines to advance our platform. We routinely draw on their expertise to meet cellular engineering challenges and they have not disappointed."
Remo Moomiaie-Qajar, Cytonus Therapeutics, Inc.
Customer Reviews
“I have had the pleasure of working with Evercyte for the last few years. We continually rely on Evercyte because of the high-quality data that they produce, their diligent responsiveness, and their excellent customer service.”
Josh Garlich, Senior Research Scientist, Apellis Pharmaceuticals, Inc.
"Cytonus has been working with Evercyte from many years as they are a trusted partner and have always delivered the highest quality cell lines to advance our platform. We routinely draw on their expertise to meet cellular engineering challenges and they have not disappointed."
Remo Moomiaie-Qajar, Cytonus Therapeutics, Inc.

