HUVEC/TERT2
Evercyte’s human umbilical vein endothelial cell line HUVEC/TERT2 can be grown without limitations while maintaining expression of cell type specific markers and function. Therefore, these cells are useful for setting up in vitro bioassays for studying angiogenesis in e.g. wound healing or metastasis as well as bioassays for testing the angiogenic properties of novel drugs. Additionally, the cell line is the perfect starting material for genetic engineering to create important disease models.
General information
Cat#: CHT-006-0008
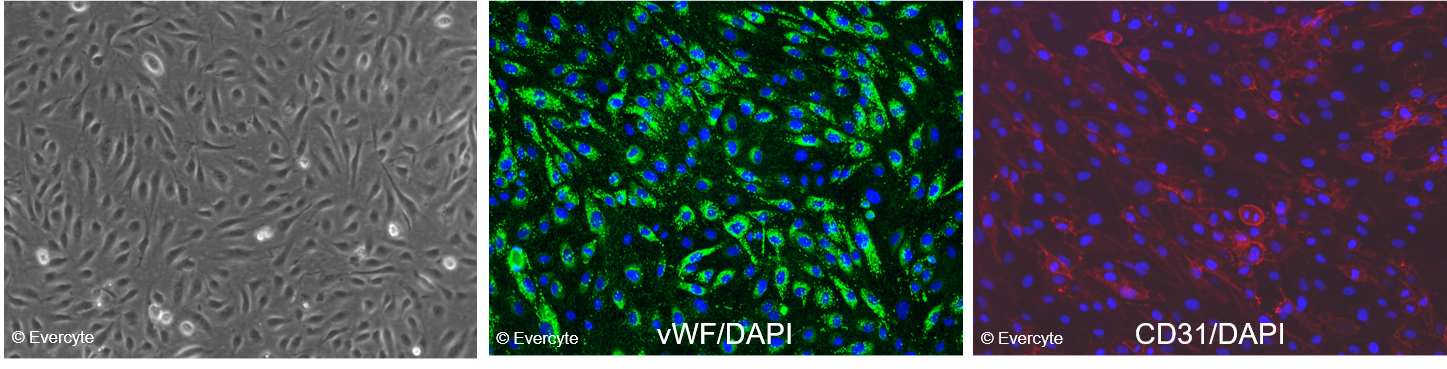
Morphology and marker expression
Response to VEGF treatment
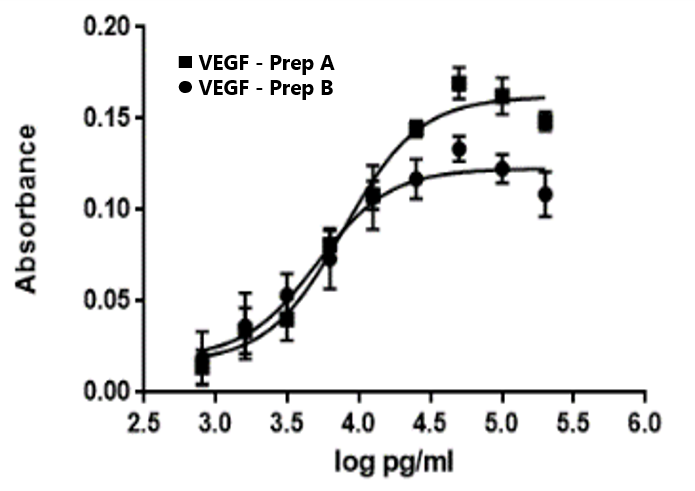
Vascular endothelial growth factor (VEGF) induces proliferation in HUVEC/TERT2 cells in a concentration dependent manner (MTT assay).
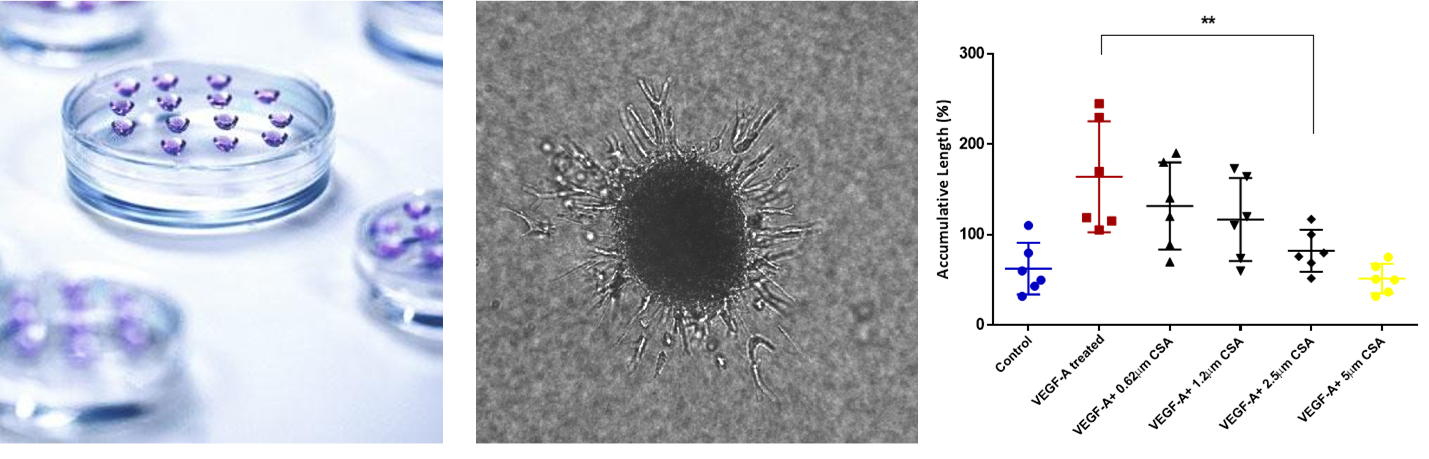
Neo-angiogenic potential
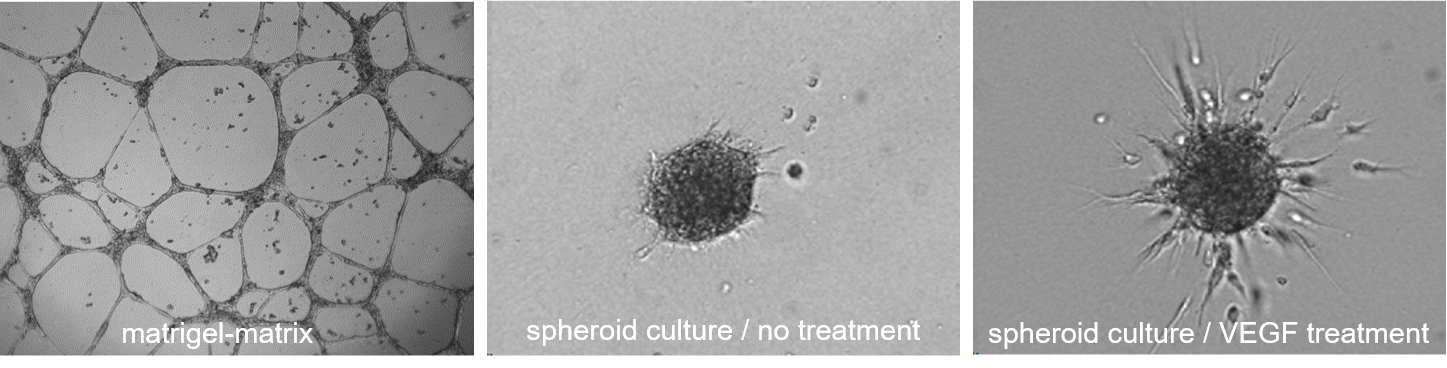
When inoculated onto Matrigel-matrix HUVEC/TERT2 cells form typical capillary like structures (left picture) demonstrating neoangiogenic potential, which is also mirrored by the formation sprouts from 3D spheroids upon VEGF treatment (right picture).
Cellular proliferation upon drug treatment
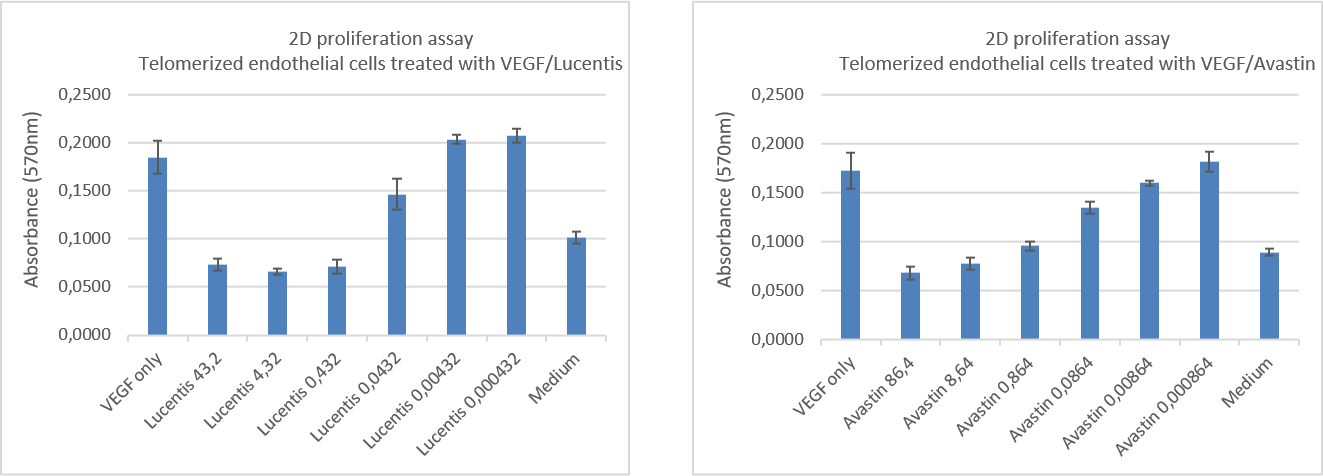
Sprout formation upon drug treatment
FAQs
In vitro propagation
Endothelial growth medium (Lonza) supplemented with EGM SingleQuot Kit, FBS and G418
EBM basal medium (Lonza, Cat#CC-3121)
Components of EGM SingleQuot Kit (Lonza, Cat# CC-4133: BBE, hEGF, hydrocortisone, ascorbic acid)
10 % FBS (PAN Biotech, Cat# P30-3031)
20 µg/ml G418 (InvivoGen, Cat# ant-gn-5)
Additional material & reagents
0,1 % Gelatin (Sigma, Cat# G1393, 2 %), diluted in PBS
Phosphate buffered saline (PBS) (Sigma, Cat# D8537)
Trypsin inhibitor (Gibco, Cat# R007100)
0,05 % Trypsin-EDTA (Gibco, Cat#25300-054)
Passaging of cells
The new culture flasks have to be pre-coated with gelatin. Therefore, the culture flasks are treated with 0.1 % gelatin solution (60 µl/cm²) at 37°C for at least 10 min (10 – 60 min). Before introducing cells, remove excess of gelatin solution.
Cryopreservation
Freezing medium
Endothelial cell growth medium (see above)
10 % DMSO (Sigma Aldrich, Cat# D2650)
Additional material & reagents
0,05 % Trypsin-EDTA (Gibco, Cat# 25300-054)
0,1 % Gelatin (Sigma, Cat# G1393, 2 %), diluted in PBS
Freezing of cells
Detach the cells from the culture vessel by using Trypsin-EDTA solution (Protocol passaging of HUVEC/TERT2).
Thawing of cells
Original Evercyte cells are to be thawed in a T25 roux flask
Product data sheet – certificate of analysis Leaflet
Protocols
Data on Markers and Functions
Selected publications
Kim BK, Lee SJ, Kim TW, Kim H, Lee H, Hong EY, Cho YJ, Kwon S, Kim SH. EGT022, an RGD-containing recombinant disintegrin, inhibits the VEGF-induced angiogenic process by targeting integrin β3 in endothelial cells. Am J Transl Res. 2023 Mar 15;15(3):1831-1841. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10086907/
Di Matteo A, Belloni E, Pradella D, Chiaravalli AM, Pini GM, Bugatti M, Alfieri R, Barzan C, Franganillo Tena E, Bione S, Terenzani E, Sessa F, Wyatt CDR, Vermi W, Ghigna C. Alternative Splicing Changes Promoted by NOVA2 Upregulation in Endothelial Cells and Relevance for Gastric Cancer. Int J Mol Sci. 2023 Apr 30;24(9):8102. doi: 10.3390/ijms24098102.
Capinha L, Zhang Y, Holzer AK, Ückert AK, Zana M, Carta G, Murphy C, Baldovini J, Mazidi Z, Grillari J, Dinnyes A, van de Water B, Leist M, Commandeur JNM, Jennings P. Transcriptomic-based evaluation of trichloroethylene glutathione and cysteine conjugates demonstrate phenotype-dependent stress responses in a panel of human in vitro models. Arch Toxicol. 2023 Feb;97(2):523-545. doi: 10.1007/s00204-022-03436-6.
Böttcher B, Pflieger A, Schumacher J, Jungnickel B, Feller K-H. 3D bioprinting of prevascularized full-thickness gelatin-alginate structures with embedded co-cultures. Bioengineering, 9(6), 242. 2022 May. https://doi.org/10.3390/bioengineering9060242
Radziwon A, Bhangu SK, Fernandes S, Cortez-Jugo C, De Rose R, Dyett B, Wojnilowicz M, Laznickova P, Fric J, Forte G, Caruso F, Cavalieri F. Triggering the nanophase separation of albumin through multivalent binding to glycogen for drug delivery in 2D and 3D multicellular constructs. Nanoscale, 14(9), 3452–3466. 2022 Jan https://doi.org/10.1039/D1NR08429A
Noël JC, et al. Quantification of selected aroma compounds in e-cigarette products and toxicity evaluation in HUVEC/Tert2 cells. Biomed Chromatogr. 2020 Mar;34(3):e4761. https://pubmed.ncbi.nlm.nih.gov/31758585
Licence Conditions
The business concept of Evercyte is to out-license telomerized cells to our customers. The license conditions depend on whether the contract partner is a for profit or a nonprofit organization and the intended use of the cells.
Nonprofit organizations
On time payment for unlimited use: EUR 1700
Profit organizations
Pharmaceutical – chemical- cosmetic industries
Contract research organizations (CRO)
Initial license fee for 3 months: EUR 2700Annual license fee R&D: royalty based
Customer Reviews
“I have had the pleasure of working with Evercyte for the last few years. We continually rely on Evercyte because of the high-quality data that they produce, their diligent responsiveness, and their excellent customer service.”
Josh Garlich, Senior Research Scientist, Apellis Pharmaceuticals, Inc.
“Cytonus has been working with Evercyte from many years as they are a trusted partner and have always delivered the highest quality cell lines to advance our platform. We routinely draw on their expertise to meet cellular engineering challenges and they have not disappointed.”
Remo Moomiaie-Qajar, Cytonus Therapeutics, Inc.
Customer Reviews
“I have had the pleasure of working with Evercyte for the last few years. We continually rely on Evercyte because of the high-quality data that they produce, their diligent responsiveness, and their excellent customer service.”
Josh Garlich, Senior Research Scientist, Apellis Pharmaceuticals, Inc.
“Cytonus has been working with Evercyte from many years as they are a trusted partner and have always delivered the highest quality cell lines to advance our platform. We routinely draw on their expertise to meet cellular engineering challenges and they have not disappointed.”
Remo Moomiaie-Qajar, Cytonus Therapeutics, Inc.

