Skin – vascular system
HDMVEC/TERT164-B
Evercyte’s human dermal microvascular endothelial cell line (lymphatic origin) HDMVEC/TERT164-B can be grown without limitations while maintaining expression of cell type-specific markers and function. Therefore, these cells are useful for setting up in vitro bioassays for studying angiogenesis in disorders involving the lymphatic system such as wound healing or metastasis as well as bioassays for testing the angiogenic properties of novel drugs. Additionally, the cell line is the perfect starting material for genetic engineering to create important disease models.
General information
Cat#: CHT-013-0164-B
Morphology and differentiation on Matrigel
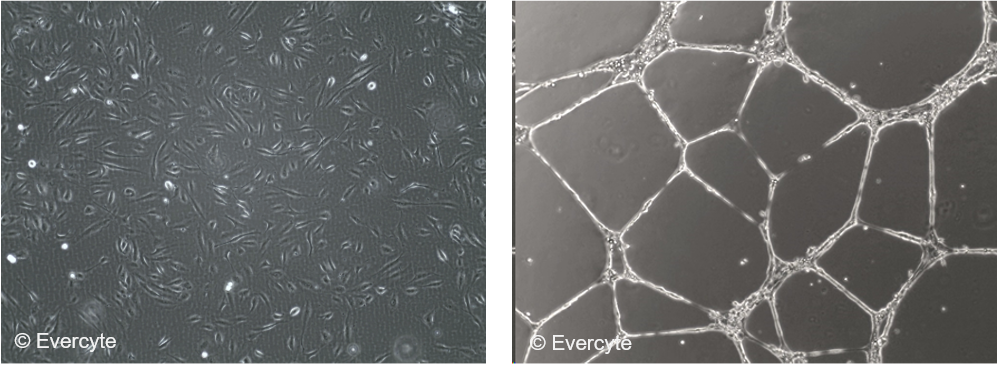
HDMVEC/TERT164-B cells can be grown for a minimum of 50 PDs with a stable growth rate. The cells are characterized by the typical endothelial morphology and form tubule-like structures when inoculated onto matrigel matrix indicating neo-angiogenic properties.
Expression of marker proteins
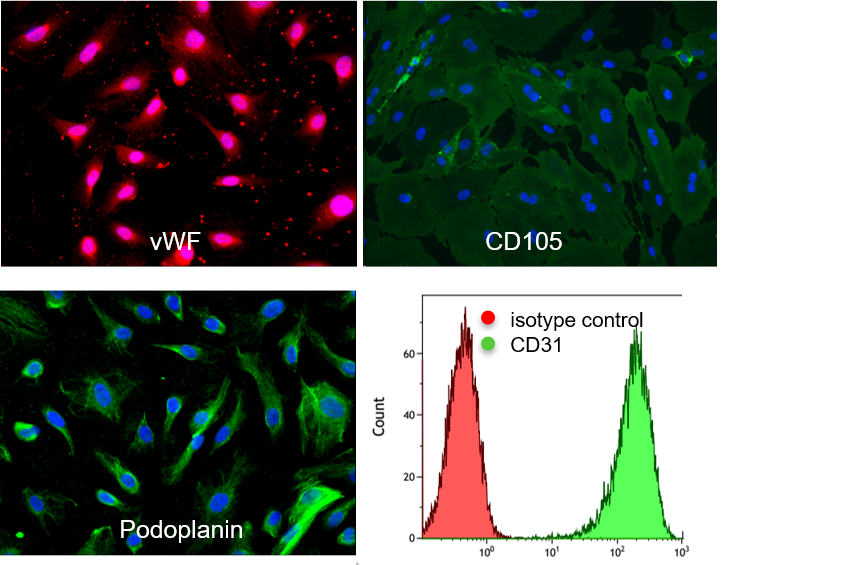
HDMVEC/TERT164-B cells homogenously express typical marker proteins of lymphatic microvascular endothelial cells such as von Willebrand factor (vWF), CD105, podoplanin and CD31 as demonstrated by immunofluorescence stainings (cell nuclei are counterstained with DAPI / left pictures; cells stained with isotype-control antibody as negative control / right picture).
PAN induced toxicity – 3D co-culture with podocytes
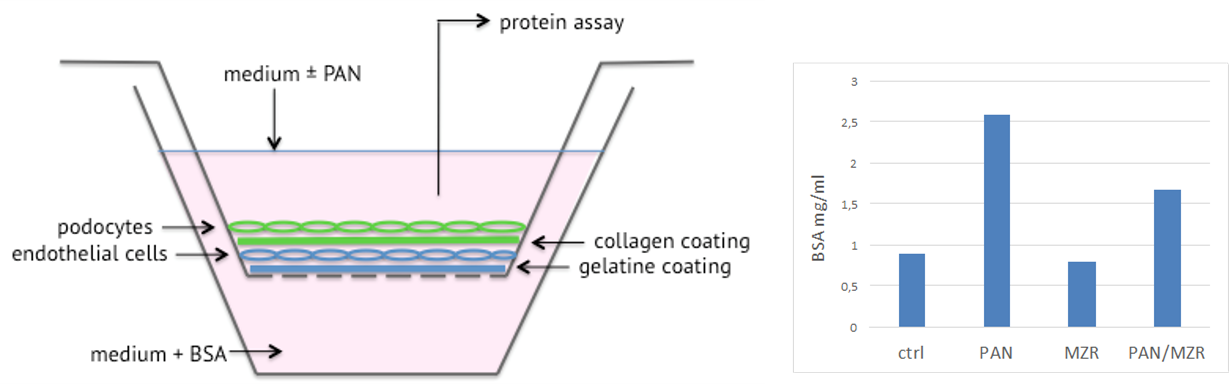
HDMVEC/TERT164 cells, co-cultured with telomerised podocytes PODO/TERT256 in transwells, form a barrier that retains bovine serum albumin (BSA); puromycin aminonucleoside (PAN) disrupts this barrier; mizoribin (MRZ) can rescue PAN induced injury.
FAQs
In vitro propagation
Endopan MV Kit (PAN Biotech) supplemented with G418
Endopan MV basalmedium (PAN Biotech, Cat# P04-0020B)
Endopan MV supplements (PAN Biotech, Cat# P04-0020S)
20 µg/ml G418 (InvivoGen, Cat# ant-gn-5)
Additional material & reagents
0,1 % Gelatin (Sigma, Cat# G1393, 2 %), dilute in PBS
Phosphate buffered saline (PBS) (Sigma, Cat# D8537)
0,05 % Trypsin-EDTA (Gibco, Cat#25300-054)
Defined Trypsin-Inhibitor (Gibco, Cat# R007100)
Passaging of cells
The new culture flasks have to be pre-coated with gelatin. Therefore, the culture flasks are treated with 0.1 % gelatin solution (80 µl/cm²) at 37°C for at least 10 min (10 – 60 min). Before introducing cells, remove excess of gelatin solution. Use the culture flask immediately for seeding the cells, the surface must not dry out.
Cryopreservation
Freezing medium
Endopan MV complete medium (PAN Biotech, Cat# P04-0020B+P04-0020S)
10 % Fetal bovine serum (Sigma Aldrich, Cat# F7524)
Additional material & reagents
Phosphate buffered saline (PBS) (Sigma, Cat# D8537)
0,1 % Gelatin (Sigma, Cat# G1393, 2 %), dilute in PBS
0,05 % Trypsin-EDTA (Gibco, Cat#25300-054)
Defined Trypsin-Inhibitor (Gibco, Cat# R007100)
Freezing of cells
Detach the cells from the culture vessel by using Trypsin-EDTA solution (Protocol passaging of HDMVEC/TERT164-B).
Thawing of cells
Original Evercyte cells are to be thawed in a T25 rouxflask
Product data sheet – certificate of analysis
Protocols
Data on Markers and Functions
Selected publications
Kaneko MK, Ohishi T, Kawada M, Kato Y. A cancer-specific anti-podocalyxin monoclonal antibody (60-mG2a-f) exerts antitumor effects in mouse xenograft models of pancreatic carcinoma. Biochem Biophys Rep. 2020 Oct 10;24:100826. doi: 10.1016/j.bbrep.2020.100826.
Gludovacz E, et al. (2020) Human diamine oxidase cellular binding and internalization in vitro and rapid clearance in vivo are not mediated by N-glycans but by heparan sulfate proteoglycan interactions. Glycobiology, cwaa090. https://pubmed.ncbi.nlm.nih.gov/32985651.
Gludovacz E, Schuetzenberger K, Resch M, Tillmann K, Petroczi K, Schosserer M, Vondra S, Vakal S, Klanert G, Pollheimer J, Salminen T A, Jilma B, Borth N, Boehm T. Heparin-binding motif mutations of human diamine oxidase allow the development of a first-in-class histamine-degrading biopharmaceutical. ELife, 10, e68542. 2021 Sep. https://doi.org/10.7554/eLife.68542
Licence Conditions
The business concept of Evercyte is to out-license telomerized cells to our customers. The license conditions depend on whether the contract partner is a for profit or a nonprofit organization and the intended use of the cells.
Nonprofit organizations
On time payment for unlimited use: EUR 1700
Profit organizations
Pharmaceutical – chemical- cosmetic industries
Contract research organizations (CRO)
Initial license fee for 3 months: EUR 2700Annual license fee R&D: royalty based
Customer Reviews
“I have had the pleasure of working with Evercyte for the last few years. We continually rely on Evercyte because of the high-quality data that they produce, their diligent responsiveness, and their excellent customer service.”
Josh Garlich, Senior Research Scientist, Apellis Pharmaceuticals, Inc.
“Cytonus has been working with Evercyte from many years as they are a trusted partner and have always delivered the highest quality cell lines to advance our platform. We routinely draw on their expertise to meet cellular engineering challenges and they have not disappointed.”
Remo Moomiaie-Qajar, Cytonus Therapeutics, Inc.
Customer Reviews
“I have had the pleasure of working with Evercyte for the last few years. We continually rely on Evercyte because of the high-quality data that they produce, their diligent responsiveness, and their excellent customer service.”
Josh Garlich, Senior Research Scientist, Apellis Pharmaceuticals, Inc.
“Cytonus has been working with Evercyte from many years as they are a trusted partner and have always delivered the highest quality cell lines to advance our platform. We routinely draw on their expertise to meet cellular engineering challenges and they have not disappointed.”
Remo Moomiaie-Qajar, Cytonus Therapeutics, Inc.

