Skin
HDF/TERT164
Evercyte’s human dermal fibroblast cell line HDF/TERT164 can be grown without limitations while maintaining expression of cell type specific markers and functions. Therefore, these cells are frequently used as standardized in vitro model to study processes such as formation of the extracellular matrix, inflammation or wound healing. Moreover, the cells can be used to established relevant and standardizable 3D skin equivalents.
General information
Cat#: CHT-008-0164
Morphology and marker expression
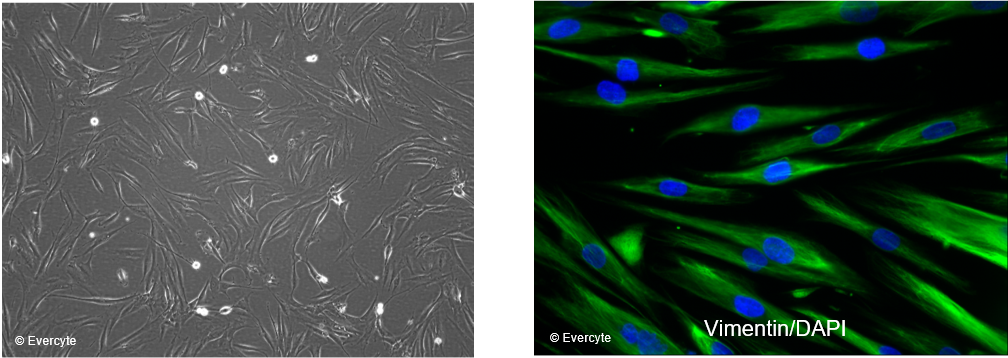
HDF/TERT164 cells are characterized by the typical, spindle shaped morphology of mesenchymal cells and homogenously express the fibroblast marker Vimentin. Cell nuclei are counterstained with DAPI.
Growth characteristics
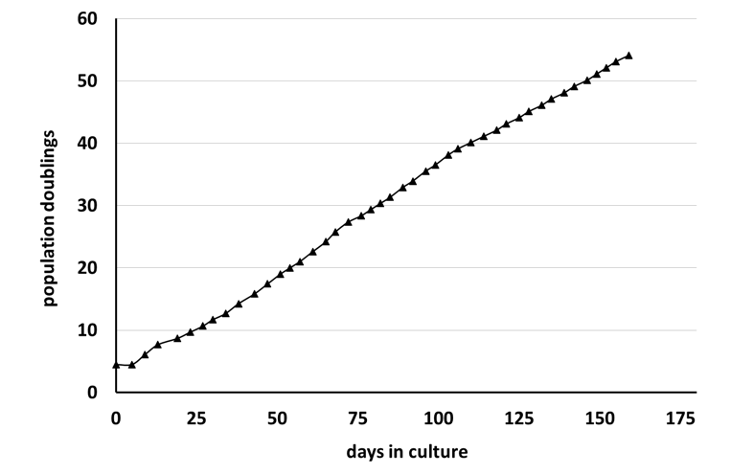
HDF/TERT164 cells can be grown with a stable growth rate for at least 50 population doublings without signs of growth retardation.
FAQs
In vitro propagation
DMEM/Ham´s F12 supplemented with Fetal Bovine Serum and G418
DMEM/Ham´s F12 (1:1) (PAN Biotech, Cat# P04-41150)
10 % FBS (PAN-Biotech, Cat#P30-3031)
Additional material & reagents
Phosphate buffered saline (PBS) (Sigma, Cat# D8537)
0,05 % Trypsin-EDTA (Gibco, Cat#25300-054)
Passaging of cells
For detachment of the cells remove and discard the culture medium and wash the cells once with PBS (about 160 µl/cm²). Remove PBS completely.
Cryopreservation
Freezing medium
10 % DMSO (Sigma Aldrich, Cat# D2650)
Additional material & reagents
0,05 % Trypsin-EDTA (Gibco, Cat#25300-054)
Freezing of cells
Detach the cells from the culture vessel by using Trypsin-EDTA solution (Protocol passaging of HDF/TERT164).
Thawing of cells
Original Evercyte cells are to be thawed in a T25 rouxflask
Product data sheet – certificate of analysis
Protocols
Data on Markers and Functions
Selected publications
Chai RJ, Wong WL, Beh CW. Developing a bioink for single-step deposition and maturation of human epidermis. Int J Bioprint. 2023 Apr 26;9(4):738. https://doi.org/10.18063/ijb.738
Hakoi H, Miki Y, Nomura S, Nakajima K, Terashima-Murase C, Takeichi T, Sano S, Akiyama M, Sakasegawa SI, Murakami M, Yamamoto K. Lysophospholipase D from Thermocrispum limits psoriatic inflammation by hydrolyzing epidermal lysoplasmalogen produced by group IIF secreted phospholipase A2. Biochimie. 2023 Dec;215:75-87. https://doi.org/10.1016/j.biochi.2023.09.027
Licence Conditions
The business concept of Evercyte is to out-license telomerized cells to our customers. The license conditions depend on whether the contract partner is a for profit or a nonprofit organization and the intended use of the cells.
Nonprofit organizations
On time payment for unlimited use: EUR 1700
Profit organizations
Pharmaceutical – chemical- cosmetic industries
Contract research organizations (CRO)
Initial license fee for 3 months: EUR 2700Annual license fee R&D: royalty based
Customer Reviews
“I have had the pleasure of working with Evercyte for the last few years. We continually rely on Evercyte because of the high-quality data that they produce, their diligent responsiveness, and their excellent customer service.”
Josh Garlich, Senior Research Scientist, Apellis Pharmaceuticals, Inc.
“Cytonus has been working with Evercyte from many years as they are a trusted partner and have always delivered the highest quality cell lines to advance our platform. We routinely draw on their expertise to meet cellular engineering challenges and they have not disappointed.”
Remo Moomiaie-Qajar, Cytonus Therapeutics, Inc.
Customer Reviews
“I have had the pleasure of working with Evercyte for the last few years. We continually rely on Evercyte because of the high-quality data that they produce, their diligent responsiveness, and their excellent customer service.”
Josh Garlich, Senior Research Scientist, Apellis Pharmaceuticals, Inc.
“Cytonus has been working with Evercyte from many years as they are a trusted partner and have always delivered the highest quality cell lines to advance our platform. We routinely draw on their expertise to meet cellular engineering challenges and they have not disappointed.”
Remo Moomiaie-Qajar, Cytonus Therapeutics, Inc.

