Skin
fHDF/TERT166
Evercyte’s human dermal fibroblast cell line fHDF/TERT166 can be grown without limitations while maintaining expression of cell type specific markers and functions. Therefore, these cells are frequently used as standardized in vitro model to study processes involving fibroblasts such as formation of the extracellular matrix, inflammation, wound healing or fibrosis. Moreover, the cells embedded into a collagen matrix and co-cultured with telomerized keratinocytes allow the establishment of standardizable 3D skin equivalents.
General information
Cat#: CHT-031-0166
Morphology and marker expression
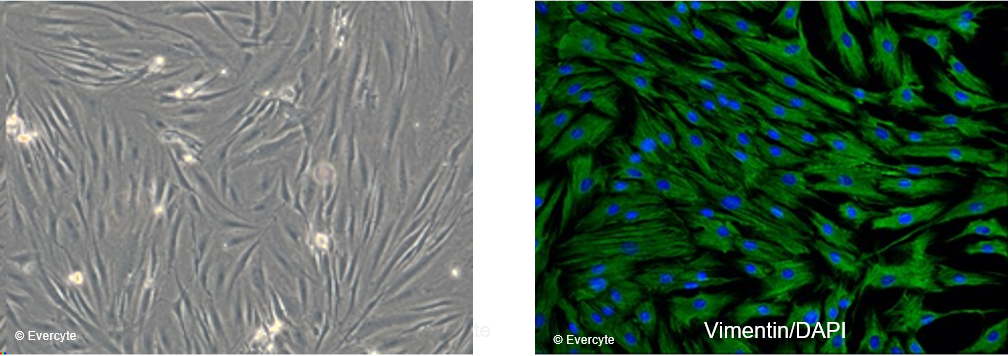
fHDF/TERT166 cells are characterized by the typical, spindle shaped morphology of mesenchymal cells and homogenously express the fibroblast marker Vimentin. Cell nuclei are counterstained with DAPI.
Response to cytokines / expression of IL-6 upon IL-17A treatment
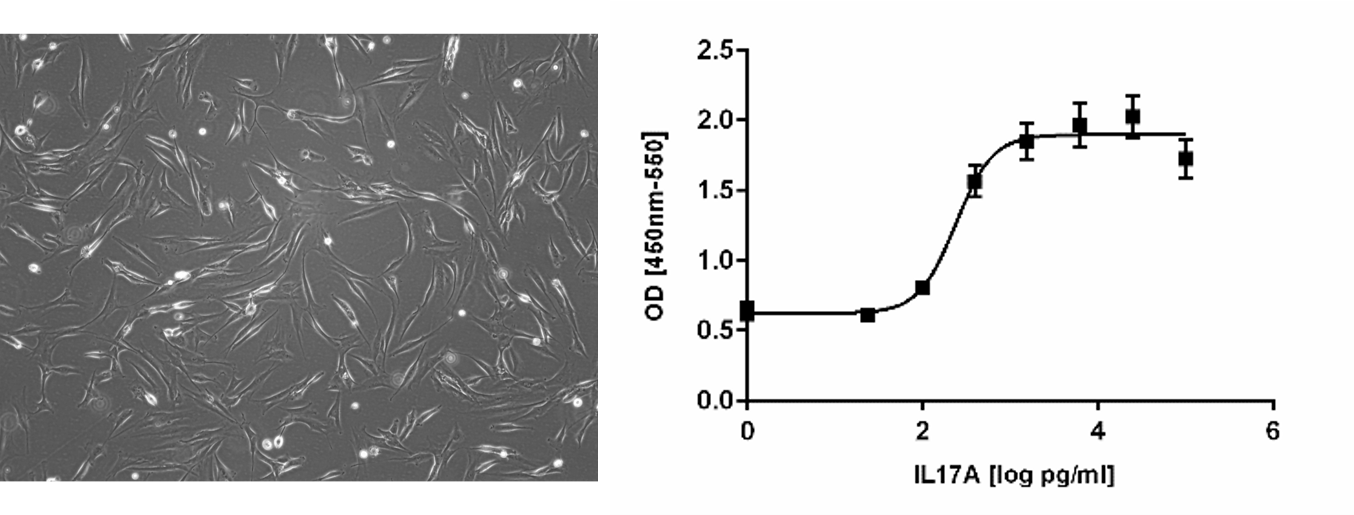
Treatment of telomerized human dermal fibroblasts fHDF/TERT166 with Interleukin-17A (IL17A) results in expression of interleukin-6 (IL6) in a concentration dependent manner.
Myofibroblast differentiation / effect of extracellular vesicles
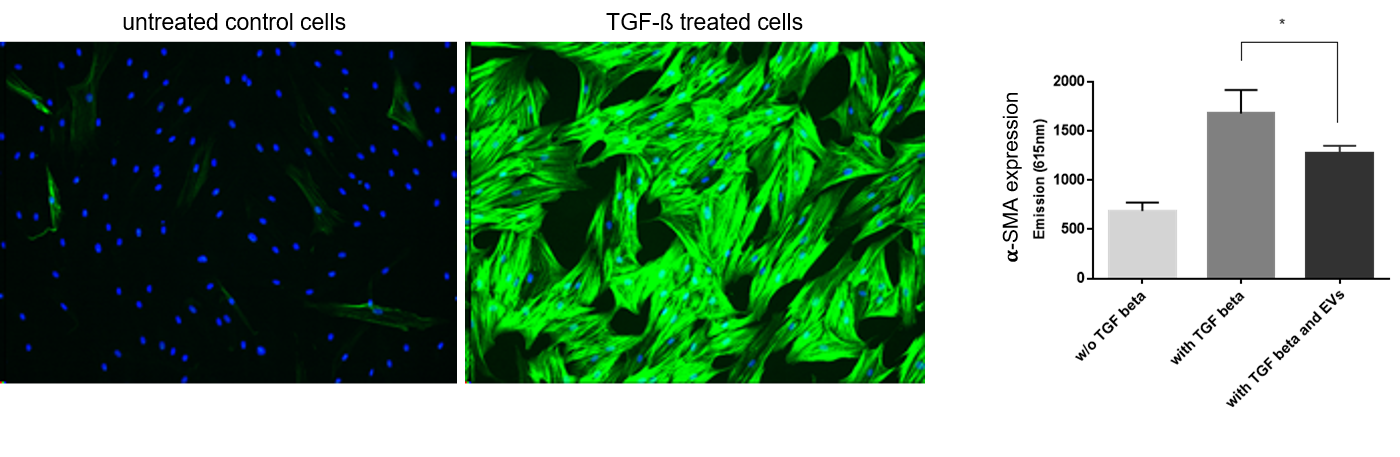
Treatment of telomerized human dermal fibroblasts fHDF/TERT166 with transforming growth factor beta (TGF-ß) induces expression of alpha smooth muscle actin (𝛂-SMA). Treatment of cells with TGF-ß together with extracellular vesicles (EVs) from mesenchymal stem cells reduces 𝛂-SMA expression significantly, demonstrating an effect of EVs on myofibroblast differentiation.
Cell migration / induction of fibroblast growth upon treatment with extracellular vesicles

fHDF/TERT166 were seeded into chamber slides and a physical gab within the monolayer was created followed by monitored the process of cell migration into the gap. Significantly less free area between the cells was detected upon addition of extracellular vesicles from mesenchymal stem cells.
FAQs
In vitro propagation
DMEM/Ham´s F12 supplemented with Fetal Bovine Serum and G418
DMEM/Ham´s F12 (1:1) (PAN Biotech, Cat# P04-41150)
10 % FBS (PAN Biotech, Cat# P30-3031)
Additional material & reagents
Phosphate buffered saline (PBS) (Sigma-Aldrich, Cat# D8537)
0,05 % Trypsin-EDTA (Gibco, Cat#25300-054)
Passaging of cells
For detachment of the cells remove and discard the culture medium and wash the cells once with PBS (about 160 µl/cm²). Remove PBS completely.
Cryopreservation
Freezing medium
DMEM/Ham´s F12 (1:1) (PAN Biotech, Cat# P04-41150)
10 % Fetal bovine serum (PAN Biotech, Cat# P30-3031)
10 % DMSO (Sigma Aldrich, Cat# D2650)
Additional material & reagents
Phosphate buffered saline (PBS) (Sigma, Cat# D8537)[ fusion_text]
Freezing of cells
Detach the cells from the culture vessel by using Trypsin-EDTA solution (Protocol passaging of fHDF/TERT166)
Thawing of cells
Original Evercyte cells are to be thawed in a T25 roux flask
Product data sheet – certificate of analysis
Protocols
Data on Markers and Functions
Selected publications
Meijer, T., Naderlinger, E., Jennings, P., & Wilmes, A. (2023). Differentiation and Subculturing of Renal Proximal Tubular-like Cells Derived from Human iPSC. Current protocols, 3(8), e850. https://doi.org/10.1002/cpz1.850
Wu, X., Chen, J., Sun, W., Hart, D. A., Ackermann, P. W., & Ahmed, A. S. (2023). Network proteomic analysis identifies inter-alpha-trypsin inhibitor heavy chain 4 during early human Achilles tendon healing as a prognostic biomarker of good long-term outcomes. Frontiers in immunology, 14, 1191536. https://doi.org/10.3389/fimmu.2023.1191536
Chen, J., Wang, J., Hart, D. A., Zhou, Z., Ackermann, P. W., & Ahmed, A. S. (2023). Complement factor D regulates collagen type I expression and fibroblast migration to enhance human tendon repair and healing outcomes. Frontiers in immunology, 14, 1225957. https://doi.org/10.3389/fimmu.2023.1225957
Chen, J., Wang, J., Wu, X. et al. eEF2 improves dense connective tissue repair and healing outcome by regulating cellular death, autophagy, apoptosis, proliferation and migration. Cell. Mol. Life Sci. 80, 128 (2023). https://doi.org/10.1007/s00018-023-04776-x
Falquet M, Prezioso C, Ludvigsen M, Bruun J-A, Passerini S, Sveinbjørnsson B, Pietropaolo V, Moens U. Regulation of Transcriptional Activity of Merkel Cell Polyomavirus Large T-Antigen by PKA-Mediated Phosphorylation. International Journal of Molecular Sciences. 2023; 24(1):895. https://doi.org/10.3390/ijms24010895
Piossek F, Beneke S, Schlichenmaier N, Mucic G, Drewitz S, Dietrich D R. Physiological oxygen and co-culture with human fibroblasts facilitate in vivo-like properties in human renal proximal tubular epithelial cells. Chemico-Biological Interactions, 361, 109959. 2022 Jul. https://doi.org/10.1016/j.cbi.2022.109959
Licence Conditions
The business concept of Evercyte is to out-license telomerized cells to our customers. The license conditions depend on whether the contract partner is a for profit or a nonprofit organization and the intended use of the cells.
Nonprofit organizations
On time payment for unlimited use: EUR 1700
Profit organizations
Pharmaceutical – chemical – cosmetic industries
Contract research organizations (CRO)
Initial license fee for 3 months: EUR 2700Annual license fee R&D: royalty based
Customer Reviews
“I have had the pleasure of working with Evercyte for the last few years. We continually rely on Evercyte because of the high-quality data that they produce, their diligent responsiveness, and their excellent customer service.”
Josh Garlich, Senior Research Scientist, Apellis Pharmaceuticals, Inc.
“Cytonus has been working with Evercyte from many years as they are a trusted partner and have always delivered the highest quality cell lines to advance our platform. We routinely draw on their expertise to meet cellular engineering challenges and they have not disappointed.”
Remo Moomiaie-Qajar, Cytonus Therapeutics, Inc.
Customer Reviews
“I have had the pleasure of working with Evercyte for the last few years. We continually rely on Evercyte because of the high-quality data that they produce, their diligent responsiveness, and their excellent customer service.”
Josh Garlich, Senior Research Scientist, Apellis Pharmaceuticals, Inc.
“Cytonus has been working with Evercyte from many years as they are a trusted partner and have always delivered the highest quality cell lines to advance our platform. We routinely draw on their expertise to meet cellular engineering challenges and they have not disappointed.”
Remo Moomiaie-Qajar, Cytonus Therapeutics, Inc.

