Extracellular vesicles
from adipose tissue-derived MSCs
Extracellular vesicles (EVs) are produced by telomerized adipose tissue-derived mesenchymal stromal cells (ASC/TERT300), and are collected from the EVscale™ perfusion culture harvests.
The EVscale™ platform, created through the collaboration of Evercyte, Phoenestra, and TAmiRNA, delivers a one-stop solution for pharmaceutical and biotech companies needing EVs at a commercial scale.
Leveraging the joint expertise of these partners, EVscale™ offers customizable projects that encompass specific cell sourcing, EV characterization, scalable production, and the correlation of EV biological functions with their RNA and protein components.
General information
Cat#: EV-001-0300
Presence of typical EV marker proteins

EVs derived from ASC/TERT300 cells carry typical surface proteins such as CD81, CD9 and CD63 as demonstrated by bead-based flow cytometry, using hCD81 positive isolation beads. Delta mean fluorescence intensity (DMFI) was calculated by normalizing to MFI of control beads.
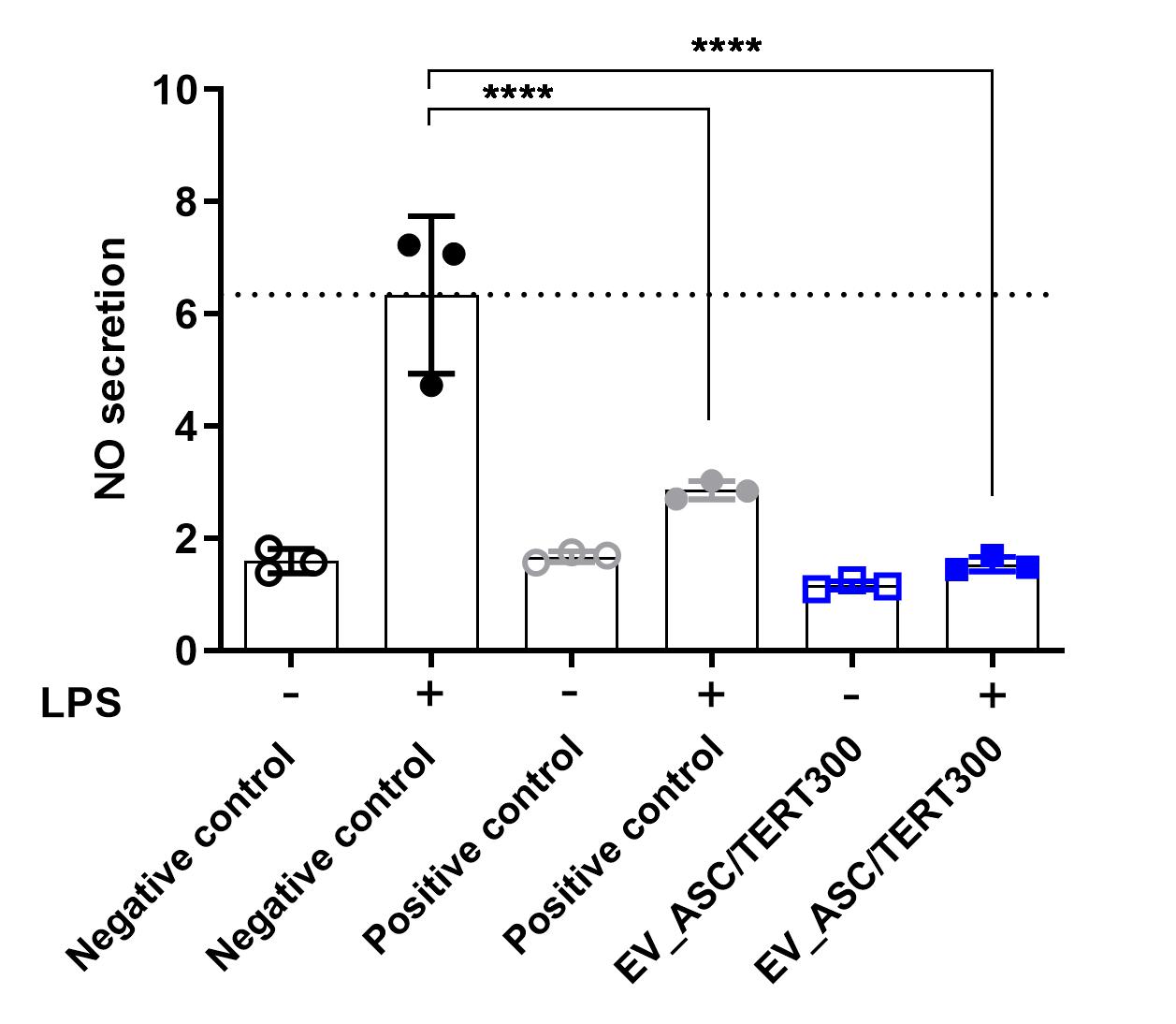
Anti-inflammatory activity of extracellular vesicles from ASC/TERT300
Treatment of mouse macrophage cells (RAW264.7) with lipopolysaccharide (LPS) induces the formation of nitric oxide (NO) indicating an inflammatory reaction. Addition of the positive control Dexamethasone significantly reduces NO secretion.
Similarly, addition of EVs derived from ASC/TERT300 cells significantly reduces NO formation indicating an anti-inflammatory activity of the EVs.
Negative control: buffer; Positive control: dexamethasone.
Anti-fibrotic activity of extracellular vesicles from ASC/TERT300
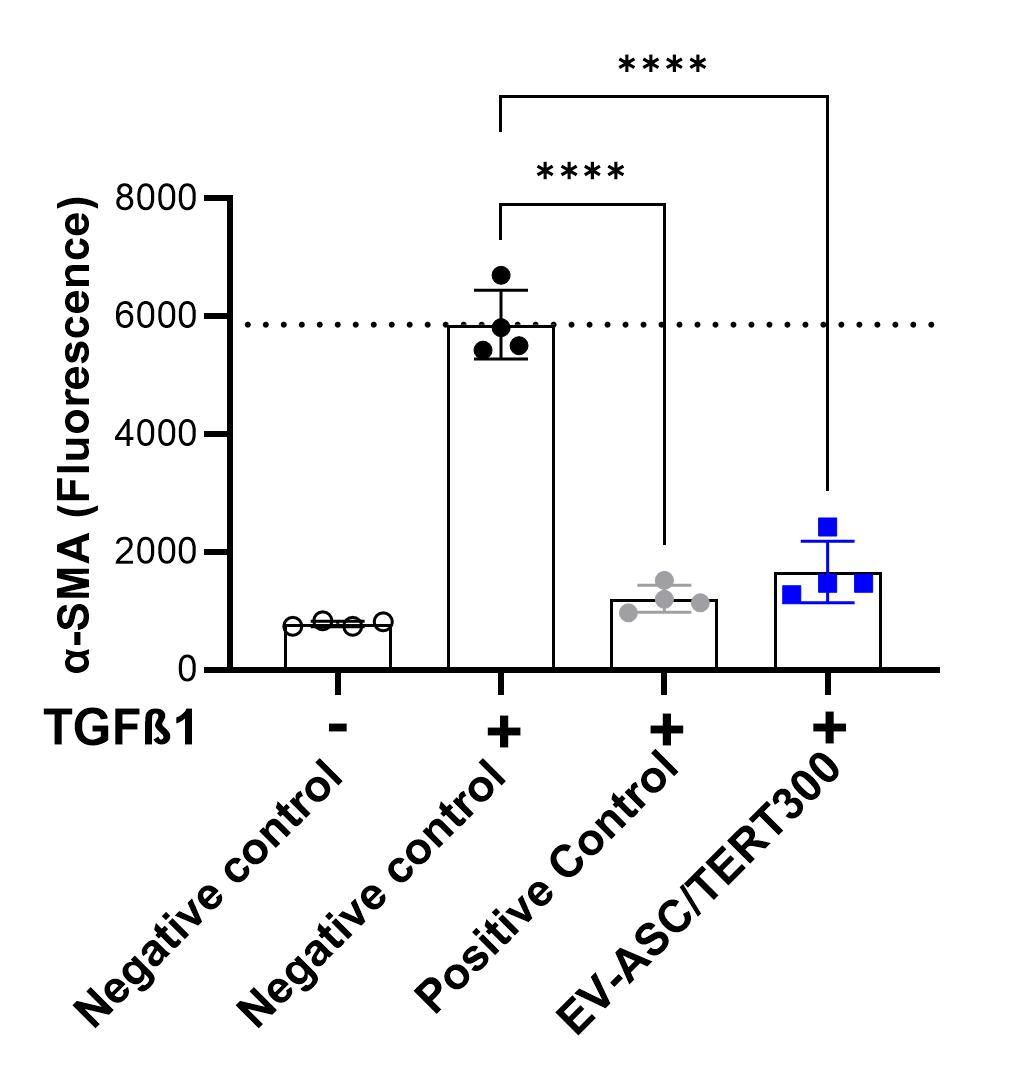
Treatment of human fibroblasts (fHDF/TERT166) with Transforming Growth Factor beta (TGF-ß1) induces the expression of alpha smooth muscle actin (α-SMA) indicating myofibroblast differentiation/activation, which is a key event in physiological and pathological tissue repair.Negative control: buffer; Positive control: kinase inhibitor
Addition of EVs derived from ASC/TERT300 cells significantly reduces α-SMA induction, indicating an anti-fibrotic activity of the EVs.
Negative control: buffer; Positive control: kinase inhibitor.
FAQs
Upon arrival immediately transfer the product to -80°C.
Store product at -80°C (for up to 6 months) until use.
Thaw the EVs on ice, centrifuge before opening the tube to ensure that the solution is collected at the bottom of the tube. Then, mix carefully by pipetting up and down and aliquot for further use to avoid multiple freeze thaw cycles.
Store the aliquots at -80°C until use.
After thawing, store the EVs at 4°C for a maximum of 1 day.
Product data sheet – certificate of analysis
Data on Markers and Functions
Applications
Selected publications: exosomes for the treatment of COVID-19
Verena Börger V, Weiss DJ, Anderson JD, Borràs FE, Bussolati B, Carter DRF, Dominici M, Falcón-Pérez JM, Gimona M, Hill AF, Hoffman AM, de Kleijn D, Levine BL, Lim R, Lötvall J, Mitsialis SA, Monguió-Tortajada M, Muraca M, Nieuwland R, Nowocin A, O’Driscoll L, Ortiz LA, Phinney DG, Reischl I, Rohde E, Sanzenbacher R, Théry C, Toh WS, Witwer KW, Lim SK, Giebel B. (2020) International Society for Extracellular Vesicles and International Society for Cell and Gene Therapy statement on extracellular vesicles from mesenchymal stromal cells and other cells: considerations for potential therapeutic agents to suppress coronavirus disease-19. Cytotherapy. 22(9):482-485. https://pubmed.ncbi.nlm.nih.gov/32425691/
Selected publications: extracellular vesicles for the treatment of wounds
Hodge JG, Robinson JL, Mellott AJ. Tailoring the secretome composition of mesenchymal stem cells to augment specific functions of epidermal regeneration: an in vitro diabetic model. Front Med Technol. 2023 Jun 12;5:1194314. doi: 10.3389/fmedt.2023.1194314. https://pubmed.ncbi.nlm.nih.gov/37378005/
Customer Reviews
“I have had the pleasure of working with Evercyte for the last few years. We continually rely on Evercyte because of the high-quality data that they produce, their diligent responsiveness, and their excellent customer service.”
Josh Garlich, Senior Research Scientist, Apellis Pharmaceuticals, Inc.
“Cytonus has been working with Evercyte from many years as they are a trusted partner and have always delivered the highest quality cell lines to advance our platform. We routinely draw on their expertise to meet cellular engineering challenges and they have not disappointed.”
Remo Moomiaie-Qajar, Cytonus Therapeutics, Inc.