General information
Cat#: CHT-001-0005
Organism: homo sapiens
Tissue, cell type: adipose tissue / liposuction (female donor), mesenchymal stromal cells
Morphology: mesenchymal morphology
Life span extension: ectopic expression of hTERT
Quality: free from contaminations (bacteria incl. mycoplasma, fungi, HIV, HAV, HBV, HCV, Parvo-B19) and cross-contaminations
Morphology and growth characteristics
ASC/TERT1 cells are characterized by the typical spindle-shaped morphology of mesenchymal stromal cells and can be grown for a minimum of 100 population doublings without showing signs of growth retardation. The cells show a constant growth rate with a population doubling time of 36-48 hours.
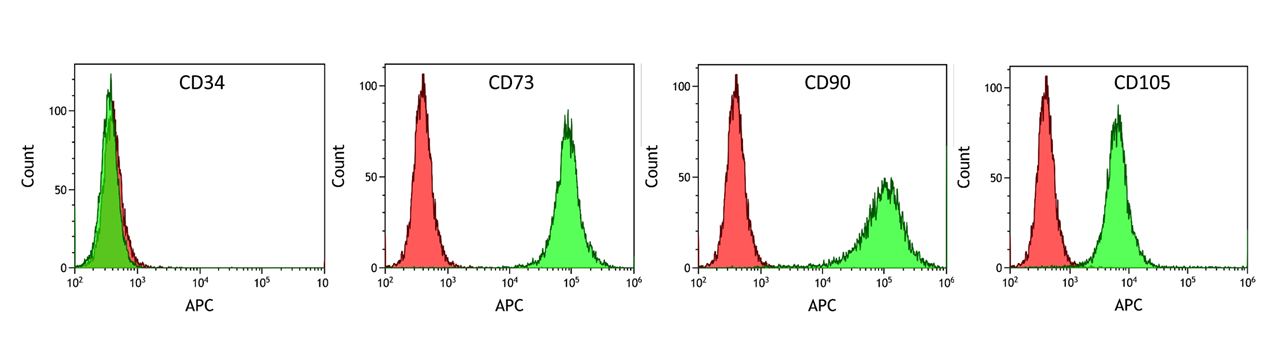
Expression of mesenchymal stromal cell marker protein
ASC/TERT1 cells homogenously express typical marker proteins of mesenchymal stromal cells such as CD73, CD90 and CD105, whereas the hematopoietic stem cell marker CD34 is not expressed (green peaks). Cells stained with isotype control antibodies are used as negative control (red peaks).
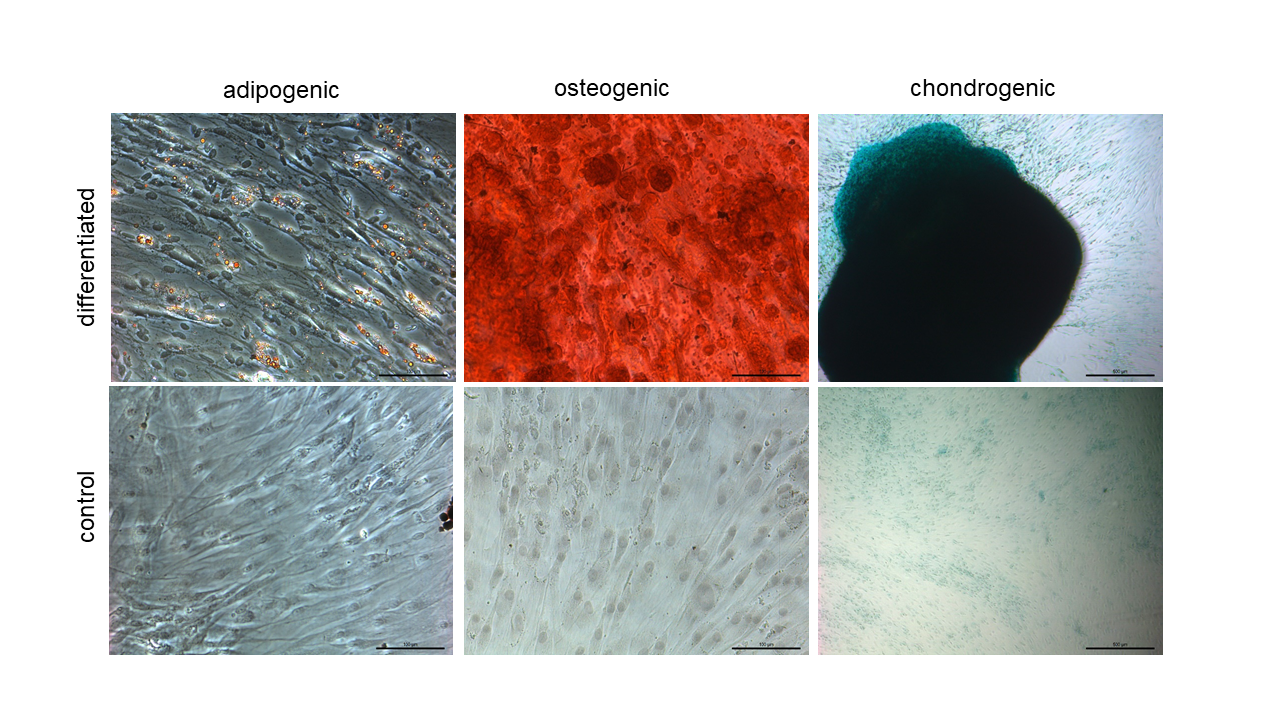
Differentiation potential towards adipocytes, osteoblasts and chondrocytes
ASC/TERT1 cells can be induced to differentiate towards adipocytes (oil red O staining), osteoblasts (Alizarin red staining) and chondrocytes ( alcian blue staining).
FAQs
What is the difference between ASC/TERT300 and ASC/TERT300-B cells?Evercyte2021-12-01T14:28:41+00:00 What is the difference between ASC/TERT1 and ASC/TERT300 cells?Evercyte2021-10-13T17:46:35+00:00
In vitro propagation
Endothelial Cell Growth Medium-2 (Lonza, Cat# CC-3162) supplemented with FBS and G418
EBM-2 basal medium (Lonza, Cat# CC-3156)
Components of EGM-2 SingleQuot Kit (Lonza, Cat# CC-4176: Hydrocortisone, hFGF, VEGF, R3-IGF-1, Ascorbic acid, hEGF, Heparin)
4 % FBS (PAN Biotech, Cat# P30-3031, ready-to-use, stored at 4°C after thawing)
200 µg/ml G418 (InvivoGen, Cat# ant-gn5)
Additional material & reagents
Phosphate buffered saline (PBS) (Sigma, Cat# D8537)
0,05 % Trypsin-EDTA (Gibco, Cat#25300-054)
Defined Trypsin-Inhibitor (Gibco, Cat# R007100)
Passaging of cells
For detachment of the cells remove and discard the culture medium and wash the cells twice with PBS (each 160 µl/cm²). Remove PBS completely.
Then, add 0.05 % Trypsin-EDTA solution (20 µl/cm²), make sure that all cells have been in contact with this solution and incubate the culture flask at 37°C for approximately 2-3 min.
Observe cell detachment under an inverted microscope. As soon as cells are detached, add Trypsin-Inhibitor (20 µl/cm²), resuspend the cells in growth medium (about 160 µl/cm²) and centrifuge at 170 g for 5 min.
Discard the supernatant, resuspend the cell pellet in the remaining droplet and add growth medium (about 160 µl/cm²).
Then, transfer appropriate aliquots of the cell suspension to new culture vessels supplemented with growth medium (final volume of 240 µl/cm²).
A split ratio of 1:3 – 1:4 twice a week is recommended (after cells have reached about 80 % confluence).
Cultivate cells at 37°C in a humidified atmosphere with 5% CO2.
Cryopreservation
Freezing medium
Complete growth medium (EGM-2 with supplements)
10 % Fetal bovine serum (PAN Biotech, Cat# P30-3031)
10 % DMSO (Sigma Aldrich, Cat# D2650)
Additional material & reagents
Phosphate buffered saline (PBS) (Sigma, Cat# D8537)
0,05 % Trypsin-EDTA (Gibco, Cat#25300-054)
Defined Trypsin-Inhibitor (Gibco, Cat# R007100)
Freezing of cells
Detach the cells from the culture vessel by using trypsin-EDTA and trypsin-inhibitor (Protocol passaging of ASC/TERT1).
Resuspend the detached cells in complete growth medium and centrifuge at 170 g for 5 min.
Discard the supernatant, resuspend the resulting cell pellet in the remaining droplet and add freezing medium (tempered to 4°C) to reach a cell density of about 5 x 105 cells/ml (for thawing in a 25 cm² culture flask).
Add 1 ml of this cell suspension to each pre-cooled cryovial and immediately transfer the cells to -80°C.
After 24 hours transfer the vials to the liquid nitrogen tank.
Thawing of cells
Original Evercyte cells are to be thawed in a T75 rouxflask
Add 6 ml of complete growth medium to a 25 cm² culture flask and place the culture flask in the incubator for at least 30 min to allow the medium to reach its normal pH.
Take a vial of frozen cells, rinse it outside with Ethanol and pre-warm in the hand until one last piece of frozen cells is seen.
Then, immediately transfer the content of the vial to a 15 ml centrifugation tube pre-filled with 9 ml of medium pre-cooled to 4°C and centrifuge for 5 min at 170 g.
Discard the supernatant and resuspend the cell pellet in the remaining droplet.
Add 1 ml of the pre-warmed medium to the cells, transfer them to the prepared culture flask and incubate at 37°C in a suitable incubator.
Perform a medium change 24 hours after thawing. If the cells are already 80 % confluent at this point, they have to be passaged.
Product data sheet – certificate of analysis
is available upon request | Please contact us indicating the respective LOT numbers
Protocols
Data on Markers and Functions
Selected publications
Promny T, Kutz C-S, Jost T, Distel LV, Kadam S, Schmid R, Arkudas A, Horch RE, Kengelbach-Weigand A. An In Vitro Approach for Investigating the Safety of Lipotransfer after Breast-Conserving Therapy. Journal of Personalized Medicine. 2022; 12(8):1284. https://doi.org/10.3390/jpm12081284
Hölzl K, Fürsatz M, Göcerler H, Schädl B, Žigon‐Branc S, Markovic M, Gahleitner C, Hoorick J V, Van Vlierberghe S, Kleiner A, Baudis S, Pauschitz A, Redl H, Ovsianikov A, Nürnberger S. Gelatin Methacryloyl as Environment for Chondrocytes and Cell Delivery to Superficial Cartilage Defects.
Journal of Tissue Engineering and Regenerative Medicine 16, no. 2 2022 Feb: 207–22.
https://doi.org/10.1002/term.3273.
Schmid R, Schmidt S K, Detsch R, Horder H, Blunk T, Schrüfer S, Schubert D W, Fischer L, Thievessen I, Heltmann‐Meyer S, Steiner D, Schneidereit D, Friedrich O, Grüneboom A, Amouei H, Wajant H, Horch R E, Bosserhoff A K, Arkudas A, & Kengelbach‐Weigand A. A New Printable Alginate/Hyaluronic Acid/Gelatin Hydrogel Suitable for Biofabrication of In Vitro and In Vivo Metastatic Melanoma Models.
Advanced Functional Materials, vol. 32, no. 2, 2021 Oct 13.
https://doi.org/10.1002/adfm.202107993.
Fürsatz M, Gerges P, Wolbank S, Nürnberger S. Autonomous spheroid formation by culture plate compartmentation.
Biofabrication. 2021 Jan 29.
https://pubmed.ncbi.nlm.nih.gov/33513590.Nürnberger S, Schneider C, Keibl C, Schädl B, Heimel P, Monforte X, Teuschl AH, Nalbach M, Thurner PJ, Grillari J, Redl H, Wolbank S. Repopulation of decellularised articular cartilage by laser-based matrix engraving.
EBioMedicine. 2021 Feb;64:103196.
https://pubmed.ncbi.nlm.nih.gov/33483297/ Dobos A, Gantner F, Markovic M, Van Hoorick J, Tytgat L, Van Vlierberghe S, Ovsianikov A. On-Chip High-Definition Bioprinting of Microvascular Structures.
Biofabrication, vol. 13, no. 1, 2020 Dec 17.
https://doi.org/10.1088/1758-5090/abb063.
Tytgat L, Dobos A, Markovic M, Van Damme L, Van Hoorick J, Bray F, Thienpont H, Ottevaere H, Dubruel P, Ovsianikov A, Van Vlierberghe S. High-Resolution 3D Bioprinting of Photo-Cross-linkable Recombinant Collagen to Serve Tissue Engineering Applications.
Biomacromolecules,
21 (10), 3997–4007. 2020 Oct.
https://doi.org/10.1021/acs.biomac.0c00386Katz DB, Huynh NPT, Savadipour A, Palte I, Guilak F. An immortalized human adipose-derived stem cell line with highly enhanced chondrogenic properties.
Biochem Biophys Res Commun. 2020 Sep 10;530(1):252-258.
https://pubmed.ncbi.nlm.nih.gov/32828295/Comas F, Latorre J, Ortega F, Oliveras-Cañellas N, Lluch A, Ricart W, Fernández-Real JM, Moreno-Navarrete JM.
Permanent cystathionine-β-Synthase gene knockdown promotes inflammation and oxidative stress in immortalized human adipose-derived mesenchymal stem cells, enhancing their adipogenic capacity.
Redox Biol. 2020 Aug 2:101668.
https://pubmed.ncbi.nlm.nih.gov/32800520/
Zerobin E, Markovic M, Tomášiková Z, Qin X, Ret D, Steinbauer P, Kitzmüller J, Steiger W, Gruber P, Ovsianikov A, Liska R, Baudis S. Hyaluronic acid vinyl esters: A toolbox toward controlling mechanical properties of hydrogels for 3D microfabrication.
Journal of Polymer Science,
58(9), 1288–1298. 2020 Mar.
https://doi.org/10.1002/pol.20200073Nürnberger S, Schneider C, van Osch G V M, Keibl C, Rieder B, Monforte X, Teuschl A H, Mühleder S, Holnthoner W, Schädl B, Gahleitner C, Redl H, Wolbank S . Repopulation of an auricular cartilage scaffold, AuriScaff, perforated with an enzyme combination.
Acta Biomaterialia,
86, 207–222. 2019 Mar. https://doi.org/10.1016/j.actbio.2018.12.035
Thul P J, Åkesson L, Wiking M, Mahdessian D, Geladaki A, Ait Blal H, Alm T, Asplund A, Björk L, Breckels L M, Bäckström A, Danielsson F, Fagerberg L, Fall J, Gatto L, Gnann C, Hober S, Hjelmare M, Johansson F, … Lundberg E. A subcellular map of the human proteome.
Science,
356(6340). 2017 May.
https://doi.org/10.1126/science.aal3321Wolbank S, Stadler G, Peterbauer A, Gillich A, Karbiener M, Streubel B, Wieser M, Katinger H, van Griensven M, Redl H, Gabriel C, Grillari J, Grillari-Voglauer R. Telomerase immortalized human amnion- and adipose-derived mesenchymal stem cells: maintenance of differentiation and immunomodulatory characteristics.
Tissue Eng Part A. 2009 Jul;15(7):1843-54.
https://pubmed.ncbi.nlm.nih.gov/19125642/
Licence Conditions
The business concept of Evercyte is to out-license telomerized cells to our customers. The license conditions depend on whether the contract partner is a for profit or a nonprofit organization and the intended use of the cells.
Nonprofit organizations
Evercyte grants licenses for an unlimited period to academic or nonprofit-organizations, whereby the use of Evercyte cell lines is restricted to research & development purposes and non-commercial use. The cells are not intended for human use.
The customers have to agree to the conditions described in our
material transfer agreement as well as accept our
general terms and conditions.
Profit organizations
Pharmaceutical – chemical- cosmetic industries
Evercyte grants licenses for commercial organizations, whereby we offer an initial testing phase for a flat fee that allows our customers to test our cells in their laboratories for a period of 6 months.
Thereafter, annual license fees fall due, depending on the cell line of interest. Besides offering cell lines for research & development purposes, we also have established cell factories that qualify for production of clinical grade extracellular vesicles for human application.
The customer has to agree to the conditions described in our license agreements.
Contract research organizations (CRO)
Evercyte grants licenses for contract research organizations, whereby we offer an initial testing phase for a flat fee that allows our customers to test our cells in their laboratories. Thereafter, we would negotiate a royalty based long-term license agreement individually.
The use of the cells during these phases is restricted to research & development purposes. The cells are not intended for human use. The customers have to agree to the conditions described in our material transfer agreement and accept our
general terms and conditions.
Initial license fee for 3 months: EUR 2700Annual license fee R&D: royalty based