Services
senolytic/
senomorphic drug testing
Senolytics, agents selectively inducing apoptosis in senescent cells that accumulate in tissues with ageing, are now considered game changers across a range of age-associated diseases, including frailty, cardiac dysfunction, vascular hyperreactivity and calcification, diabetes mellitus, liver steatosis, osteoporosis, vertebral disk degeneration, and pulmonary fibrosis. Senolytic agents are being tested in several proof-of-concept clinical trials.
Increased focus on senolytics and senomorphics/senostatics (suppression of senescence markers or preventing secondary senescence), brings the need for new tools to identify novel agents and elucidate their modes of action, including drug screening with relevant and standardizable in vitro models. Evercyte offers a fast-track development route from initial senolytic candidate hit to lead cell-based testing.
General information
In vitro model systems offered: Evercyte ´s senolytic efficacy and toxicity assays are based on human cells from different tissues and organs (e.g. skin fibroblasts, lung fibroblasts, umbilical vein endothelial cells, microvascular endothelial cells, renal proximal tubular epithelial cells, mesenchymal stem cells).
Induction of senescence in human normal cells
characterization of the senescent phenotype
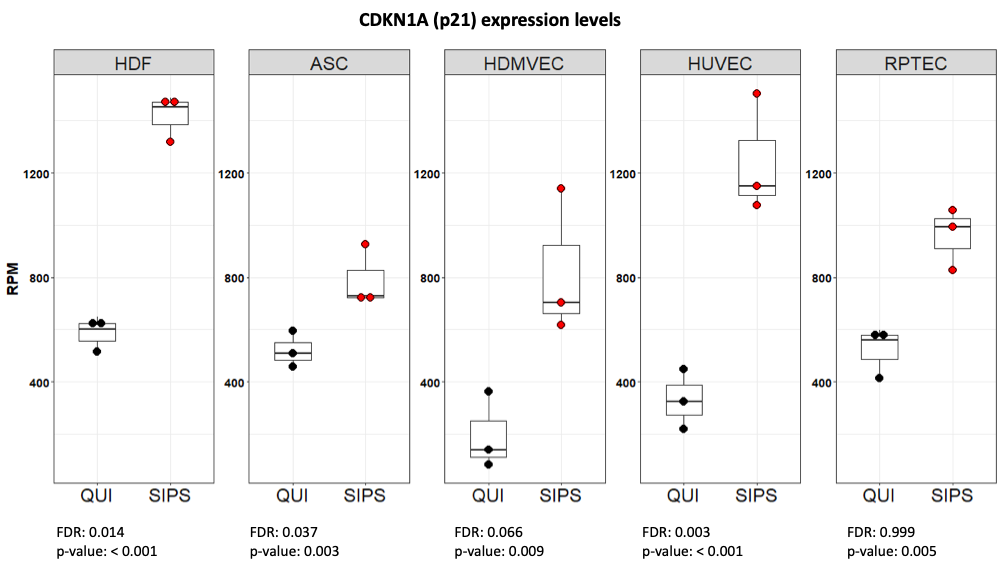
Normal human cells are treated with doxorubicin following established protocols to induce stress induced premature senescence (SIPS). Thereafter, the senescent phenotype is confirmed by transcriptomic analysis. As shown in above figure, a significant increase in p21 in SIPS compared to quiescent cells (QUI) is observed in all cell types analysed.
HDF: human dermal fibroblasts, ASC: adipose-derived mesenchymal stem cells, HDMVEC: human dermal microvascular endothelial cells, HUVEC: human umbilical vein endothelial cells, RPTEC: renal proximal tubular epithelial cells.
Data have been generated in cooperation with TAmiRNA.
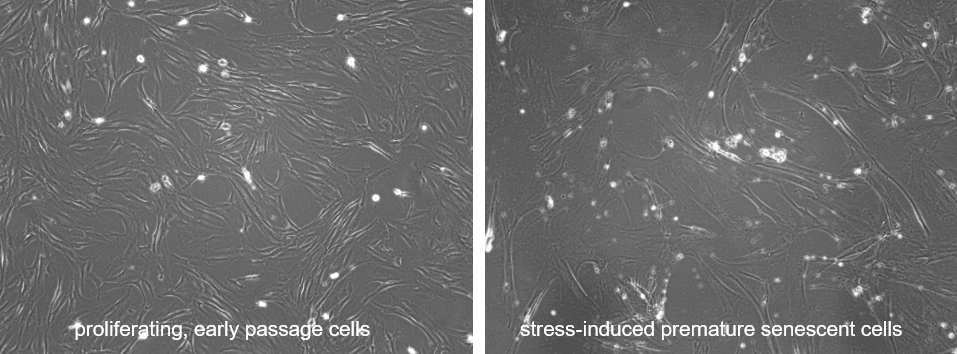
Normal human lung fibroblasts are treated with doxorubicin following established protocols to induce stress induced premature senescence (SIPS). Whereas normal, early passage, subconfluent cells are characterized by the typical spindle-shaped mesenchymal morphology, doxorubicin treated cells aquire the typical, flattened, multi-nucleated morphology of senescent cells.
Testing senolytic activity of compounds
measurement of cellular viability

Human quiescent and senescent (SIPS) skin fibroblasts are treated with compounds and cellular viability is measured after 3 days. This allows us to identify compounds with a potential senolytic (blue) activity against senescent cells as well as cytotoxic activity (gray) against growth arrested control cells.
Customer Reviews
“I have had the pleasure of working with Evercyte for the last few years. We continually rely on Evercyte because of the high-quality data that they produce, their diligent responsiveness, and their excellent customer service.”
Josh Garlich, Senior Research Scientist, Apellis Pharmaceuticals, Inc.
"Cytonus has been working with Evercyte from many years as they are a trusted partner and have always delivered the highest quality cell lines to advance our platform. We routinely draw on their expertise to meet cellular engineering challenges and they have not disappointed."
Remo Moomiaie-Qajar, Cytonus Therapeutics, Inc.
Customer Reviews
“I have had the pleasure of working with Evercyte for the last few years. We continually rely on Evercyte because of the high-quality data that they produce, their diligent responsiveness, and their excellent customer service.”
Josh Garlich, Senior Research Scientist, Apellis Pharmaceuticals, Inc.
"Cytonus has been working with Evercyte from many years as they are a trusted partner and have always delivered the highest quality cell lines to advance our platform. We routinely draw on their expertise to meet cellular engineering challenges and they have not disappointed."
Remo Moomiaie-Qajar, Cytonus Therapeutics, Inc.