Extracellular vesicles
from Wharton´s Jelly MSCs
Extracellular vesicles (EVs) play an essential role in cellular communication by transporting proteins, lipids as well as nucleic acids. EVs from our Wharton´s Jelly derived mesenchymal stem cells (WJ-MSC/TERT273) are characterized by the typical EV morphology, presence of EV marker proteins as well as anti-inflammatory, anti-fibrotic, neo-angiogenic and wound healing activities.
General information
Cat#: EV-059-0273-A1 (1 x 10^9 EVs in 100 µl buffer), EV-059-0273-A2 (5 x 10^9 EVs in 50 µl buffer)
Presence of typical EV marker proteins
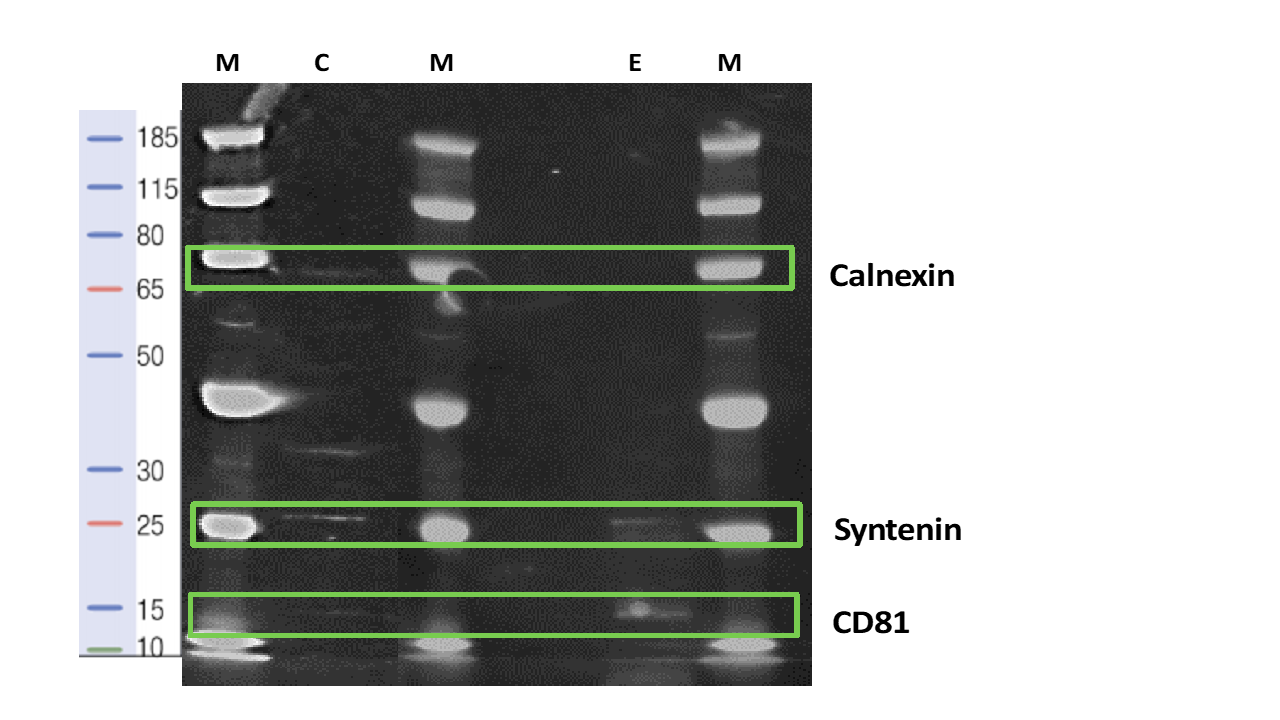
EVs derived from WJ-MSC/TERT273 cells carry typical surface proteins such as CD81 and Syntenin. EVs do not carry Calnexin as shown by western blotting.
Morphology of extracellular vesicles from WJ-MSC/TERT273
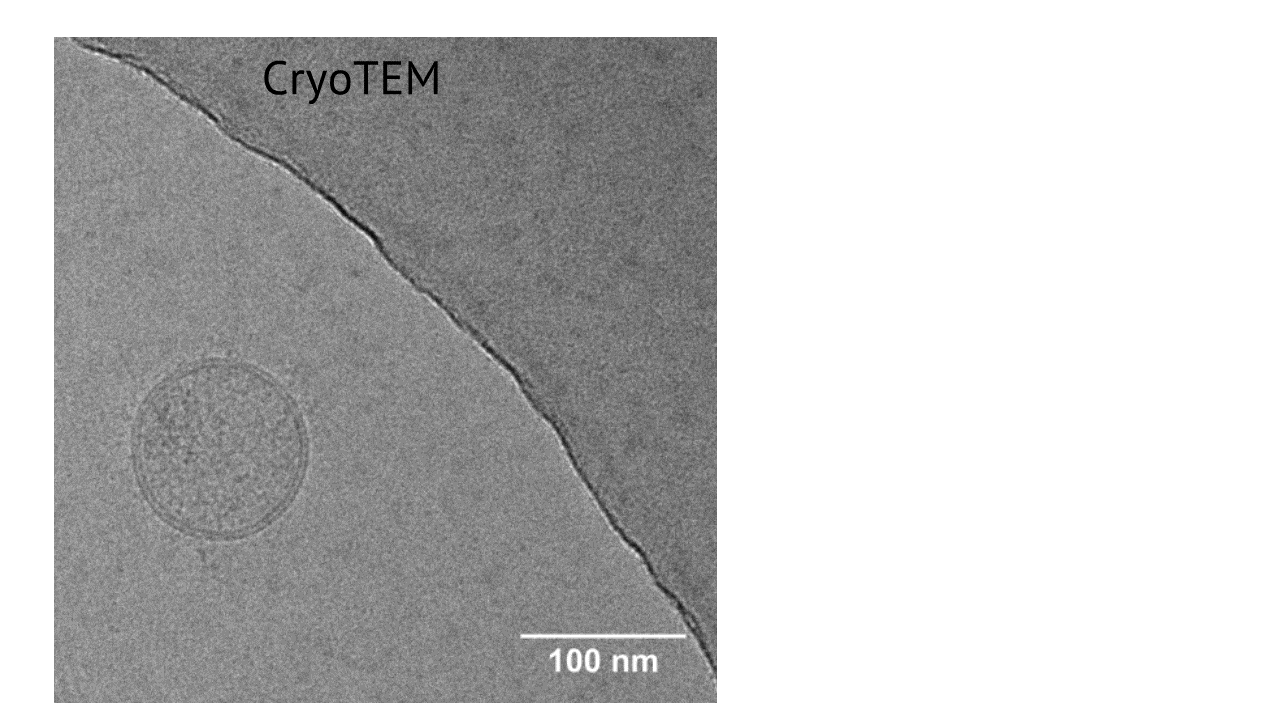
EVs derived from WJ-MSC/TERT273 cells show the typical EV morphology with lipide double layer membrane as demonstrated by cryo electron microscopy (cryo EM).
Anti-inflammatory activity of extracellular vesicles from WJ-MSC/TERT273
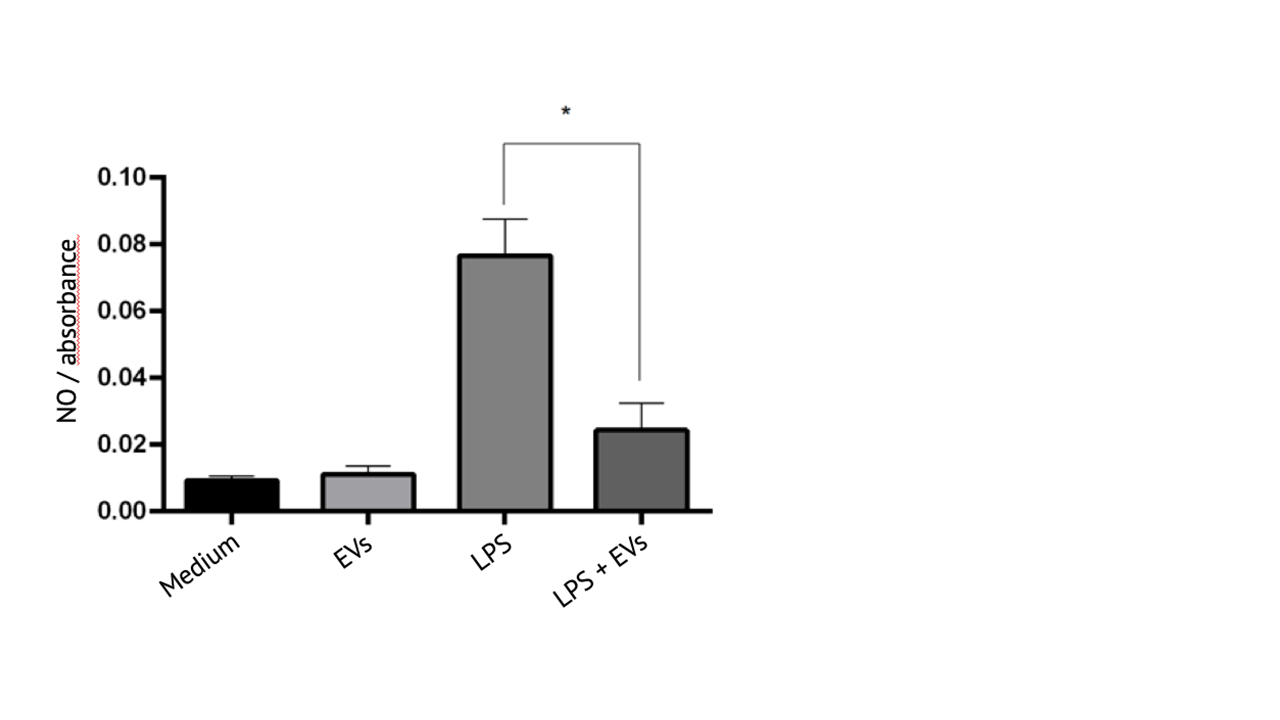
Treatment of mouse macrophage cells (RAW264.7) with lipopolysaccharide (LPS) induces the formation of nitric oxide (NO) formation indicating an inflammatory reaction.
Addition of extracellular vesicles from Wharton´s Jelly derived mesenchymal stem cells significantly reduces NO formation demonstrating anti-inflammatory activity of extracellular vesicles from Wharton´s Jelly derived mesenchymal stem cells in vitro.
Anti-fibrotic activity of extracellular vesicles from WJ-MSC/TERT273
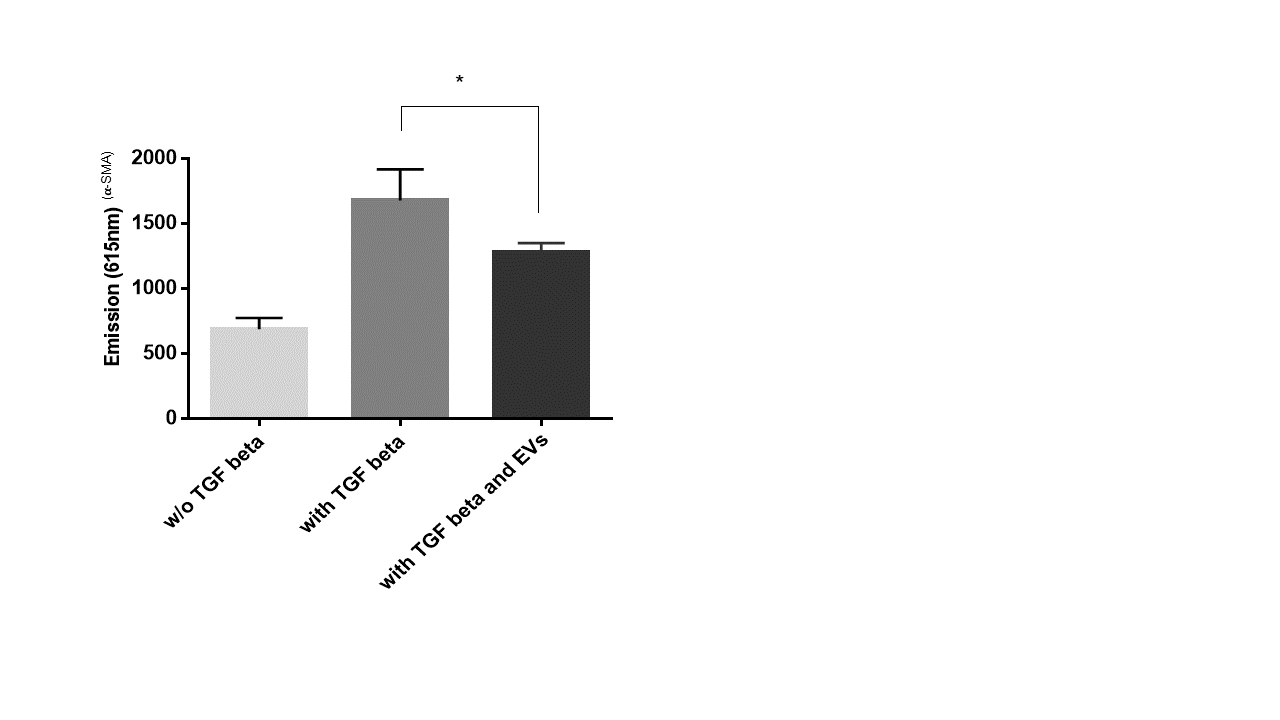
Treatment of human fibroblasts (fHDF/TERT166) with Transforming Growth Factor beta (TGF-ß) induces the expression of alpha smooth muscle actin (𝛂-SMA) indicating myofibroblast differentiation/activation, which is a key event in physiological and pathological tissue repair.
Addition of extracellular vesicles from Wharton´s Jelly derived mesenchymal stem cells significantly reduces expression of 𝛂-SMA indicating anti-fibrotic activity of extracellular vesicles from Wharton´s Jelly derived mesenchymal stem cells in vitro.
Fibroblast growth promoting activity of extracellular vesicles from WJ-MSC/TERT273
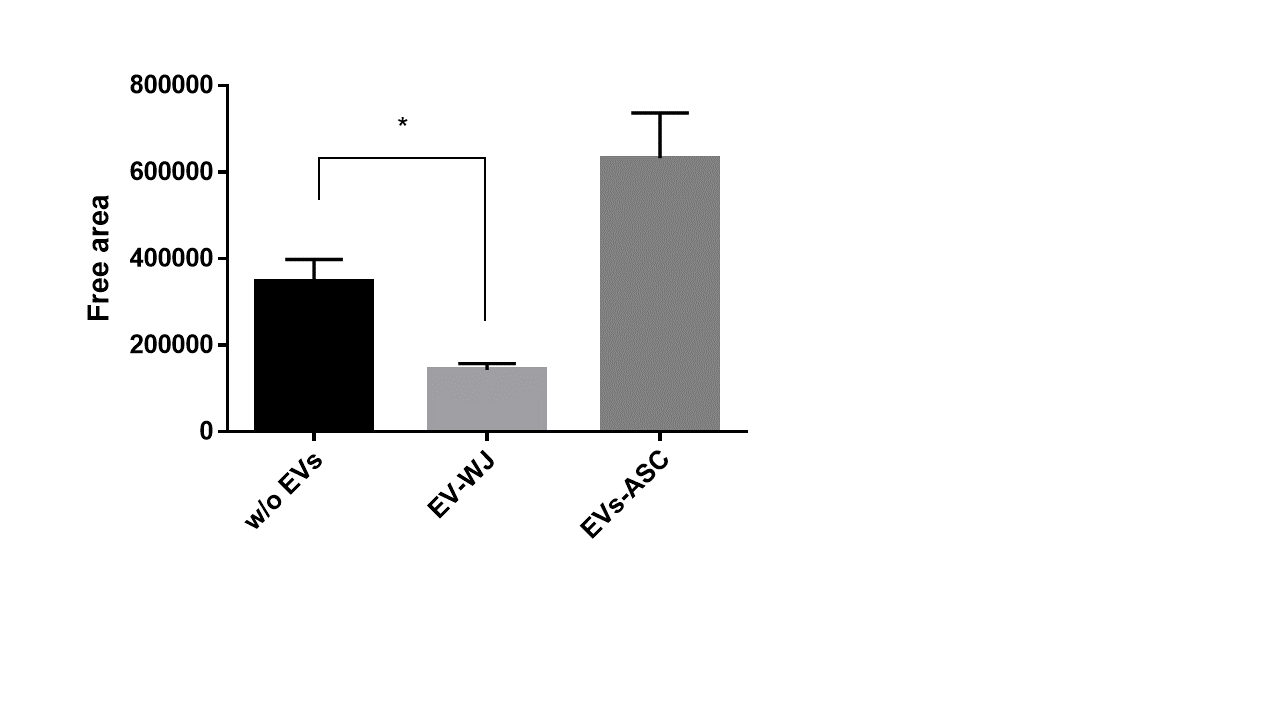
Treatment of human fibroblasts (fHDF/TERT166), grown in an in vitro wound healing / scratch assay with extracellular vesicles from Wharton´s Jelly derived MSCs (EV-WJ) induces cell migration even better as control medium (w/o EVs). On the contrary, exosomes derived from adipose-derived MSCs (Evs-ASC) did not induce fibroblast growth.
These data indicate fibroblast growth promoting / wound healing activity of extracellular vesicles from Wharton´s Jelly derived mesenchymal stem cells.
Neo-angiogenic potential of extracellular vesicles from WJ-MSC/TERT273
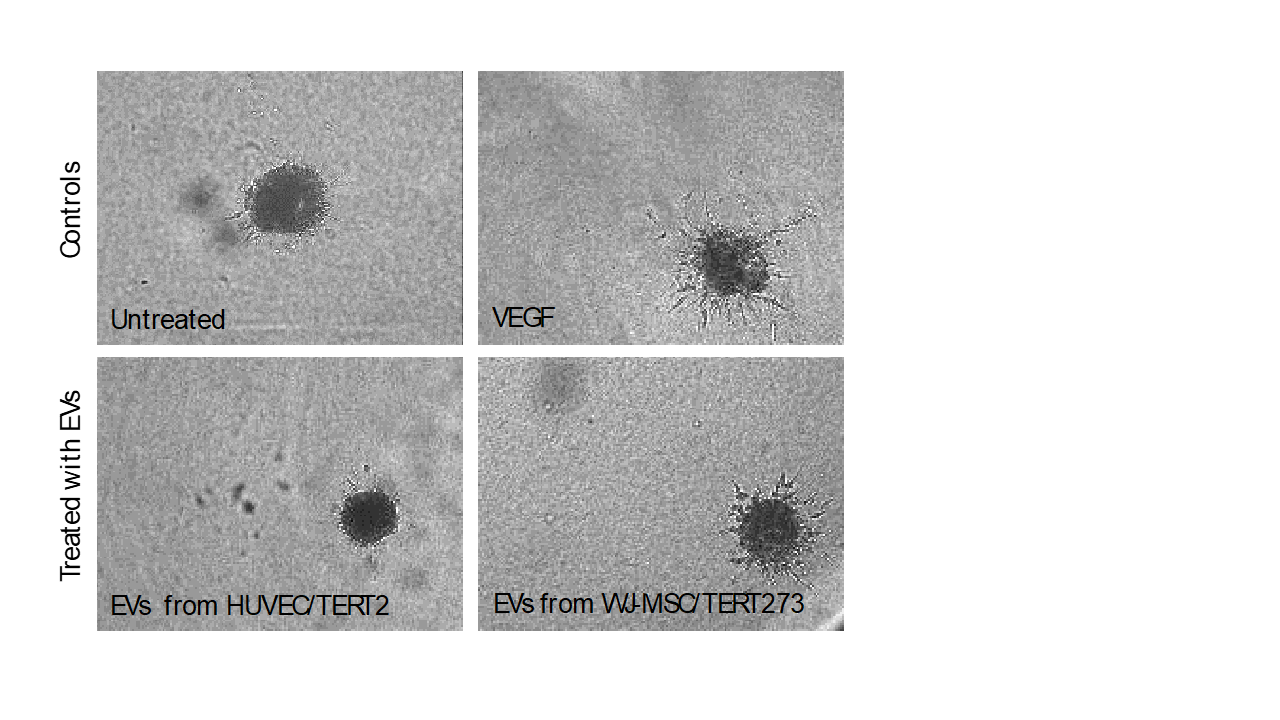
Treatment of endothelial spheroids with vascular endothelial growth factor (VEGF) induces the formation of sprouts indicating neo-angiogenic potential.
A similar effect is detected when spheroids are treated with extracellular vesicles derived from our Wharton´s Jelly derived mesenchymal stem cells WJ-MSC/TERT273.
No sprout formation is detected when spheroids are treated with EVs from endothelial cells HUVEC/TERT2.
Counteracting anti-angiogenic effect of drugs
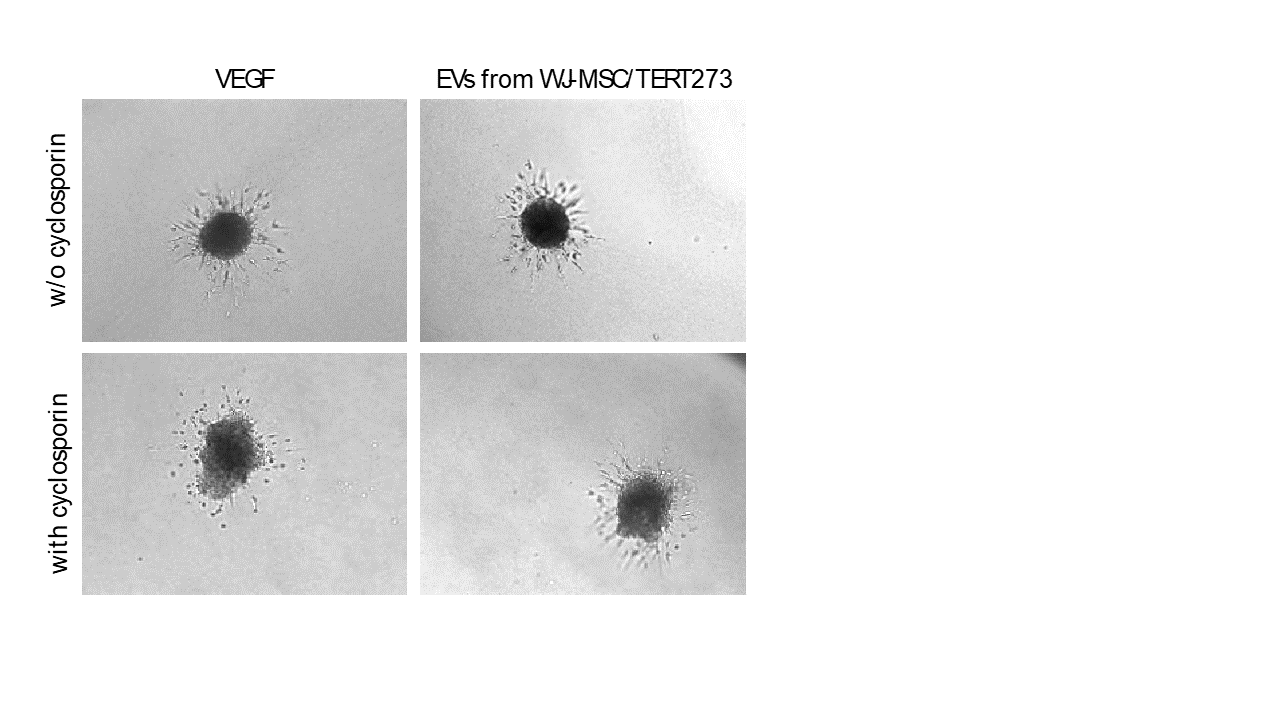
Treatment of endothelial spheroids with vascular endothelial growth factor (VEGF) induces the formation of sprouts, whereby sprout formation is inhibited upon treatment with cyclosporin A.
Anti-angiogenic effect of cyclosporin A is inhibited by addition of extracellular vesicles from Wharton´s Jelly mesenchymal stem cells.
miRNA cargo of extracellular vesicles from WJ-MSC/TERT273
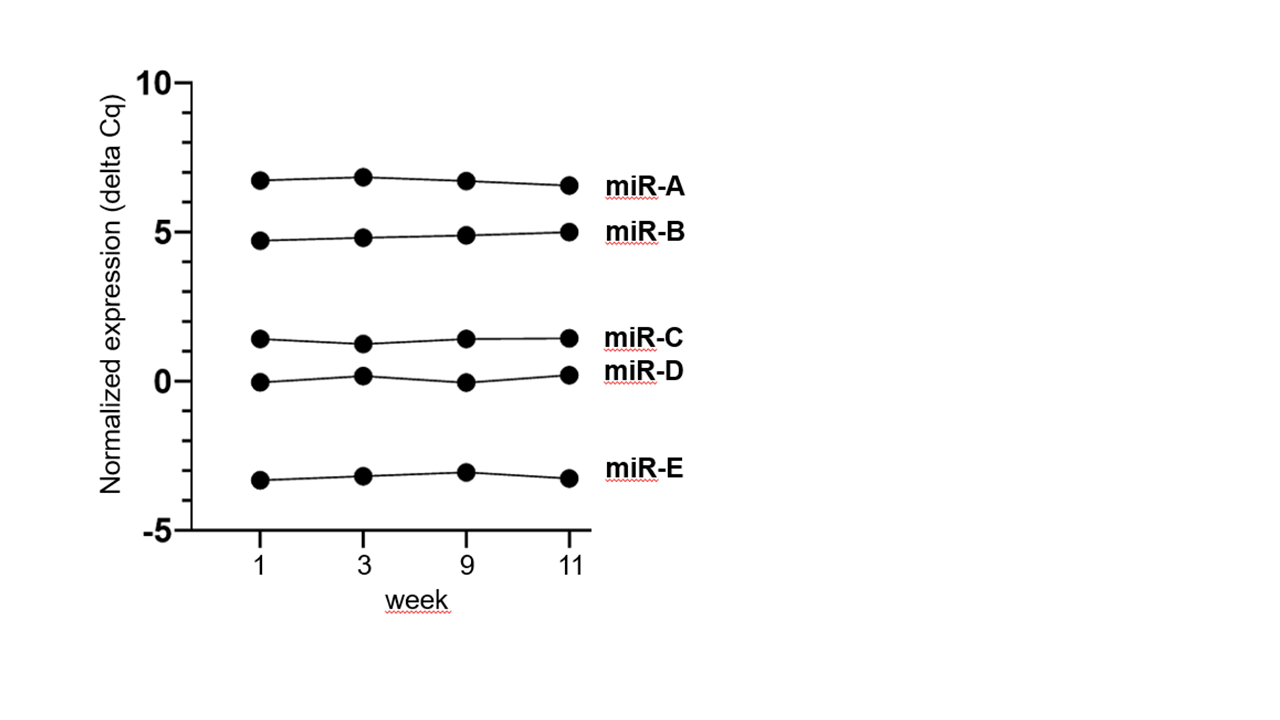
Extracellular vesicles were produced using a hollow fiber bioreactor, whereby supernatants were harvested 1-2 times per week over a period of 3-4 months.
After enrichment of particles by tangential flow filtration (TFF) and RNA isolation, a panel of microRNAs was analyzed.
During the whole production process the EV cargo of analyzed miRNAs is very stable.
FAQs
Upon arrival immediately transfer the product to -80°C.
Store product at -80°C (for up to 6 months) until use.
Thaw the EVs on ice, centrifuge before opening the tube to ensure that the solution is collected at the bottom of the tube. Then, mix carefully by pipetting up and down and aliquot for further use to avoid multiple freeze thaw cycles.
Store the aliquots at -80°C until use.
After thawing, store the EVs at 4°C for a maximum of 1 day.
Product data sheet – certificate of analysis
Data on Markers and Functions
Applications
Selected publications: exosomes for the treatment of COVID-19
Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N. (2020) Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020 Jun 15;29(12):747-754. https://pubmed.ncbi.nlm.nih.gov/32425691/
Selected publications: exosomes for the treatment of wounds
Casado-Díaz A, Quesada-Gómez JM, Dorado G. (2020) Extracellular Vesicles Derived From Mesenchymal Stem Cells (MSC) in Regenerative Medicine: Applications in Skin Wound Healing. Front Bioeng Biotechnol. 8:146. https://pubmed.ncbi.nlm.nih.gov/32195233/
Customer Reviews
“I have had the pleasure of working with Evercyte for the last few years. We continually rely on Evercyte because of the high-quality data that they produce, their diligent responsiveness, and their excellent customer service.”
Josh Garlich, Senior Research Scientist, Apellis Pharmaceuticals, Inc.
“Cytonus has been working with Evercyte from many years as they are a trusted partner and have always delivered the highest quality cell lines to advance our platform. We routinely draw on their expertise to meet cellular engineering challenges and they have not disappointed.”
Remo Moomiaie-Qajar, Cytonus Therapeutics, Inc.
Customer Reviews
“I have had the pleasure of working with Evercyte for the last few years. We continually rely on Evercyte because of the high-quality data that they produce, their diligent responsiveness, and their excellent customer service.”
Josh Garlich, Senior Research Scientist, Apellis Pharmaceuticals, Inc.
“Cytonus has been working with Evercyte from many years as they are a trusted partner and have always delivered the highest quality cell lines to advance our platform. We routinely draw on their expertise to meet cellular engineering challenges and they have not disappointed.”
Remo Moomiaie-Qajar, Cytonus Therapeutics, Inc.


