Cornea
hTCEpi
The cornea is constantly exposed to the outside environment and thus subject to damage due to various insults such as infections, drug- or chemical induced toxicity. In order to sustain such damage, the corneal epithelial cells form a resistance barrier, which is continuously renewed by stem cells located in the adjacent limbal region.
Evercyte offers a continuously growing corneal epithelial cell line with functions that resemble that of the corresponding primary counterpart cells. Therefore, our cells qualify for studying drug transport, drug- and chemical-induced toxicity on the corneal epithelium as well as inflammation processes and wound healing.
General information
Cat#: CHT-045-0237
Morphology and expression of marker proteins
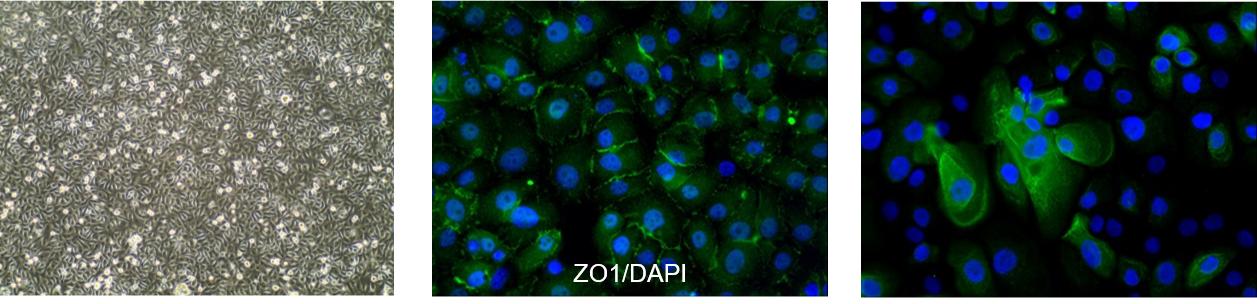
hTCEpi cells are characterized by the typical epithelial cobblestone appearance and expression of corneal epithelial cell markers such as ZO1 and KRT3.
FAQs
In vitro propagation
KGM™️ Gold Keratinocyte Growth Medium BulletKitTM (Lonza)
Additional material & reagents
Phosphate buffered saline (PBS) (Sigma, Cat# D8537)
Trypsin inhibitor (Gibco, Cat# R007100)
0,05 % Trypsin-EDTA (Gibco, Cat#25300-054)
Passaging of cells
For detachment of the cells remove and discard the culture medium and wash the cells once with PBS (about 160 µl/cm²). Remove PBS completely.
Cryopreservation
Freezing medium
CryoStor® cell cryopreservation medium CS10 (Sigma Aldrich, Cat# C2874)
Additional material & reagents
Phosphate buffered saline (PBS) (Sigma, Cat# D8537)
0,05 % Trypsin-EDTA (Gibco, Cat# 25300-054)
Trypsin inhibitor (Gibco, Cat# R007100)
Freezing of cells
Detach the cells from the culture vessel by using Trypsin-EDTA and Trypsin-Inhibitor (see Protocol passaging of hTCEpi).
Thawing of cells
Original Evercyte cells are to be thawed in a T5 rouxflask.
Product data sheet – certificate of analysis
Protocols
Data on Markers and Functions
Selected publications
Raghunathan V, Edwards SG, Leonard BC, Kim S, Evashenk AT, Song Y, Rewinski E, Marangakis Price A, Hoehn A, Chang C, Reilly CM, Muppala S, Murphy CJ, Thomasy SM. Differential effects of Hsp90 inhibition on corneal cells in vitro and in vivo. Exp Eye Res. 2021 Jan;202:108362. https://pubmed.ncbi.nlm.nih.gov/33220237/
Kasus-Jacobi A, Land CA, Stock AJ, Washburn JL, Pereira HA. Antimicrobial Peptides Derived from the Immune Defense Protein CAP37 Inhibit TLR4 Activation by S100A9. Invest Ophthalmol Vis Sci. 2020 Apr 9;61(4):16. https://pubmed.ncbi.nlm.nih.gov/32298435/
Alekseev O, Donegan WE, Donovan KR, Limonnik V, Azizkhan-Clifford J. HSV-1 Hijacks the Host DNA Damage Response in Corneal Epithelial Cells through ICP4-Mediated Activation of ATM. Invest Ophthalmol Vis Sci. 2020 Jun 3;61(6):39. https://pubmed.ncbi.nlm.nih.gov/32543665/
Kim S, Gates B, Leonard BC, Gragg M, Pinkerton KE, Winkle LV, Murphy CJ, Pyrgiotakis G, Zhang Z, Demokritou P, Thomasy SM. Engineered metal oxide nanomaterials inhibit corneal epithelial wound healing in vitro and in vivo. NanoImpact. 2020 Jan;17:100198. https://pubmed.ncbi.nlm.nih.gov/32154443/
Robertson DM, Li L, Fisher S, Pearce VP, Shay JW, Wright WE, Cavanagh HD, Jester JV. Characterization of growth and differentiation in a telomerase-immortalized human corneal epithelial cell line. Invest Ophthalmol Vis Sci. 2005 Feb;46(2):470-8. https://pubmed.ncbi.nlm.nih.gov/15671271/
Licence Conditions
The business concept of Evercyte is to out-license telomerized cells to our customers. The license conditions depend on whether the contract partner is a for profit or a nonprofit organization and the intended use of the cells.
Nonprofit organizations
On time payment for unlimited use: EUR 1700
Profit organizations
Pharmaceutical – chemical- cosmetic industries
Contract research organizations (CRO)
Initial license fee for 3 months: EUR 2700Annual license fee R&D: royalty based
Customer Reviews
“I have had the pleasure of working with Evercyte for the last few years. We continually rely on Evercyte because of the high-quality data that they produce, their diligent responsiveness, and their excellent customer service.”
Josh Garlich, Senior Research Scientist, Apellis Pharmaceuticals, Inc.
“Cytonus has been working with Evercyte from many years as they are a trusted partner and have always delivered the highest quality cell lines to advance our platform. We routinely draw on their expertise to meet cellular engineering challenges and they have not disappointed.”
Remo Moomiaie-Qajar, Cytonus Therapeutics, Inc.

